By Jamshed Arslan, Pharm. D., PhD.
Since its inception in 1979, Western blotting has undergone several developments. The use of radioactive probes was common throughout 1980s, but utilizing secondary antibodies labelled with fluorescent/chemiluminescent probes has virtually removed the need for radioactivity. Western blotting has become a routine quantitative method in biological research and immunodiagnostics. The following primer discusses some considerations when quantitating Western blot data.
Know the sample properties
When determining how a protein of interest changes during aging, treatment or disease, the sample’s inherent properties cannot be ignored. For example, total protein used to normalize the relative abundance of your protein of interest will be different for a normal/healthy tissue than a fibrotic/infarcted one. In other words, you are bound to get a decreased expression of a muscle-specific structural protein of interest from a diseased tissue compared to a healthy one.
Know your antibody specificity
Antibodies can bind to similar molecular weight protein(s) non-specifically, as it happens sometimes during the analysis of G protein-coupled receptors. To avoid this, you must consider the catalog number of an antibody, since vendors often sell multiple antibodies recognizing the same protein target. Use positive and negative controls recommended by the same vendor and try to identify any non-specific bands you see on the blot. When replicating a study, keep in mind the antibody dilution and host, since they also impact the performance of an antibody.
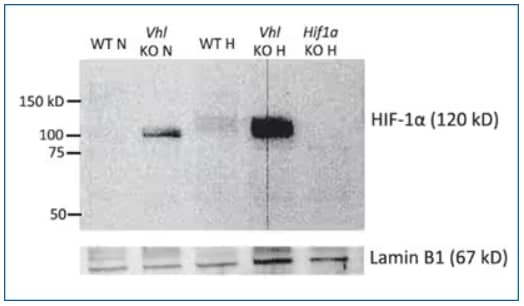
Biological Strategies Validation and Genetic Strategies Validation. Western Blot: HIF-1 alpha Antibody (H1alpha67) [NB100-105] - Naive CD4 T cells from WT, VHL-deficient (Vhl KO), or HIF-1a-deficient (Hif1a KO) mice were differentiated under IL-22-skewing conditions for a total of 60 h. Some cells remained at normoxia for the duration of the culture (N); others were at normoxia for 35 h and then hypoxia (1% O2) for 24 h (H). At 60 h, nuclear extracts were harvested, and HIF-1a and Lamin B1 levels were analyzed by Western blot.
Recommended Positive Control
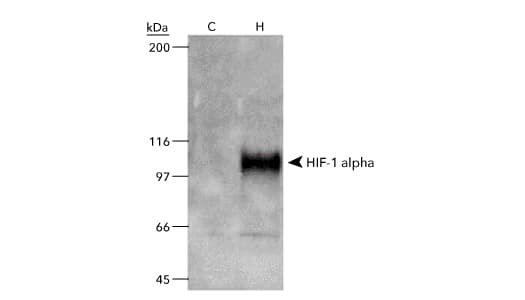
COS-7 Nuclear Hypoxic Induced Cell Lysate [NB800-PC26] - WB analysis of 50ug nuclear lysate of COS7 cells which were left untreated (C) or were treated with Cobalt Chloride / CoCl2 (H) before preparation of lysates (catalog# NB800-PC26). The blot was developed using HIF-1 alpha antibody (clone H1alpha67; catalog# NB100-105).
Recommended Negative Control
HIF-1 alpha Knockout HeLa Cell Lysate-Knockout achieved by using CRISPR/Cas9,8 bp deletion in exon 3 of HIF1A gene.
Take care of your samples!
Freezing and thawing can degrade or mask the epitope. Consequently, the relative abundance of your protein of interest generally may appear reduced relative to a freshly prepared sample.
Use proper loading conditions and loading controls
When using a well-characterized antibody, it is unnecessary to fractionate the tissue even when the amount of the protein target is low in your sample. Sample enrichment can lead to loss of some protein of interest, which might place quantitation in jeopardy. A lowly expressed protein of interest can often be detected better with sensitive chemiluminescence at the end of a Western blot protocol than by loading more amount of protein. Many researchers opt for single-cell Western approaches to avoid saturation and consequent non-linearity during analysis.
This approach applies to loading controls like housekeeping proteins (GAPDH or actin) as well. The frequently used loading amount (10-50 ug) of cell lysate is the saturating quantity for GAPDH and actin. It is impossible to quantitate these loading control proteins through immunodetection since they are typically not in the same linear range of detection as the protein of interest.
Harness the power of Western blotting through best practices
Western blot is a reliable quantitative method only if sample properties and integrity, antibody specificity to the target protein, and loading protocols are considered. With careful attention to details, you can avoid common mistakes and avoid misinterpreting Western blot data.

Jamshed Arslan, Pharm D, PhD
Dr Arslan is an Assistant Professor at Dow University of Health Sciences, Pakistan,
where he teaches Pharmacology to future pharmacists.
-
Bass, J.J. et al. (2017) An Overview of Technical Considerations for Western Blotting Applications to Physiological Research Scandinavian Journal of Medicine & Science in Sports 1:4-25.
-
McDonough, Alicia A. et al. (2014) Considerations when Quantitating Protein Abundance by Immunoblot American Journal of Physiology, Cell Physiology 6:C426-C433.
-
Murphy, Robyn M., & Graham D. Lamb (2013) Important Considerations for Protein Analyses using Antibody Based Techniques: Down-Sizing Western Blotting Up-Sizes Outcomes Journal of Physiology 23:5823-5831.