By Michalina Hanzel, PhD
In this final instalment of our three blog-posts series on major cellular mechanisms responsible for neurodegenerative disorders, we will explore the processes of neuroinflammation and microglial activation. Previously, the role of autophagy in the clearance of aggregation-prone proteins in the context of neurodegenerative diseases was discussed in Mechanisms of Neurodegeneration: Protein aggregation and failure of autophagy, and the role of mitochondria and reactive oxygen species in neurodegenerative diseases was discussed in Mechanisms of Neurodegeneration: Mitochondrial dysfunction and oxidative stress.
Microglia: a 'double-edged sword' in brain health and neurodegeneration
Another important cellular mechanism that contributes to neurodegenerative disorders is neuroinflammation that results from aberrant chronic activation of microglia, the brain-resident immune cells. Microglia are indispensable for normal brain development and homeostatic functions, performing various important roles, from the protection and maintenance of synapses to phagocytosis of debris and regulating CNS immune responses. In a healthy brain, microglia adopt a 'resting' state with a ramified morphology and dynamic processes used to monitor the microenvironment. Therefore, microglia often assume useful roles at the beginning of a disease, yet progress into a dysfunctional phenotype as the disease advances and the cells' metabolic responses change. In a chronically activated state, microglia may actively contribute to disease progression as they exacerbate the inflammatory environment by enhanced secretion of inflammatory cytokines, ineffective phagocytosis of proteins and neuronal debris, as well as metabolic disturbances.
Neuroinflammation in Alzheimer's disease
Neuroinflammation has only recently been recognized as a causative factor in neurodegeneration, not a mere consequence of the disease. In Alzheimer's disease (AD), genetic and bioinformatic studies have shown that immune system activation occurs prior to AD onset. Interestingly, patients with mutations in genes encoding immune system proteins (for example TREM2 and CD33) and those with high systemic levels of inflammation due to unrelated diseases (such as obesity or psoriasis) have an increased risk of developing AD, suggesting a strong link between inflammation and neurodegeneration.
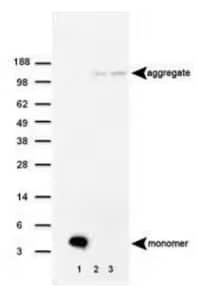
|
Western Blot: beta Amyloid Antibody (MOAB-2) [NBP2-13075] - Analysis of beta Amyloid (MOAB-2) antibody in (1) 100 pmole beta Amyloid 42, (2) 5xFAD mouse brain homogenate Repetition 1 and (3) 5xFAD mouse brain homogenate Repetition 2. |
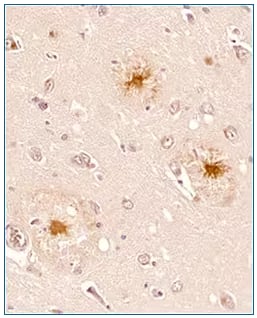
|
Immunohistochemistry-Paraffin: beta Amyloid Antibody (MOAB-2) [NBP2-13075] - IHC analysis of a formalin fixed paraffin embedded tissue section of human brain (Alzheimer’s disease, hippocampus) using 1:200 dilution of anti-beta Amyloid antibody (clone MOAB-2). The staining was developed with HRP labeled anti-mouse secondary antibody and DAB reagent, and nuclei of cells were counter-stained with haematoxylin. This beta Amyloid antibody specifically stained the cells with Abeta 42/ Abeta aggregates while the normal cells were negative for abeta peptide. |
During disease progression, Amyloid beta (Aβ) exhibits various forms (from monomers, oligomers and protofibrils to plaques) that interact with microglia via receptor- and non-receptor mediated interactions. Recent research implies that Aβ deposition has a 'priming' effect on brain microglia, resulting in reactive cells more susceptible to activation by secondary stimuli. Aβ presence sustains chronic activation of primed microglia leading to overproduction of cytokines and chemokines, which, in turn, exacerbate microglial activation further. Ultimately, microglial overactivation contributes to neuronal loss and progression of the disease. Similar mechanisms have been proposed to play a role in other neurodegenerative diseases such as Parkinson's disease and Huntington's disease. Common therapeutic interventions targeting microglial dysregulation might therefore be beneficial at early stages of many neurodegenerative disorders.
Microglia as a potential therapeutic target
Because microglia produce both neuroprotective, as well as deleterious effects on the neuronal microenvironment, any therapeutic intervention will need to modulate microglial activation to promote the former and suppress the latter outcomes. New models will be needed to best address these questions. For example, organotypic brain slice cultures, new animal models and iPS cells will all help decipher the complex relationship between microglia, peripheral inflammatory stimuli and neurons. Researchers and doctors need to learn from previous unsuccessful attempts at immunotherapies in the context of neurodegeneration and proceed with cautious optimism as ongoing research reveals more and more details of microglial function. This might lead to a discovery of a common microglial activation pathway that could potentially be targeted with a therapeutic agent in several neurodegenerative diseases simultaneously.
Michalina Hanzel, PhD
Postdoctoral Associate at The Rockefeller University
Dr Hanzel is currently studying synaptic function in the cerebellum to understand neurodevelopmental disorders and has a background in developmental neurobiology, molecular and cell biology.
-
Aldana, B. I. (2019) Microglia-Specific Metabolic Changes in Neurodegeneration Journal of Molecular Biology
-
Hammond, T. R. et al. (2019) Immune Signaling in Neurodegeneration Immunity
-
Haneka, M. et al. (2015) Neuroinflammation in Alzheimer’s disease Dementia, Fifth Edition
-
Heppner, F. L. et al. (2015) Immune attack: the role of inflammation in Alzheimer disease Nature Reviews. Neuroscience
-
Ransohoff, R. M. (2016) How neuroinflammation contributes to neurodegeneration Science