By Jennifer Jones, M.S.
Spheroids and organoids are two words that, like “butter” and “margarine”, are often referred to interchangeably but have distinct meanings. The progression and adoption of three-dimensional (3D) cell culture models, such as spheroids and organoids, from traditional two-dimensional (2D) cell culture has helped researchers to better understand complex biological processes.1-7 Both models provide a platform for mimicking the function and structure of tissues and organs in vitro. Here, we discuss the unique features of 3D spheroids and organoids and their advantages and disadvantages to help you choose the best model for your research.
Spheroids: The Original 3D Cell Culture
First developed in the 1970s, spheroids are generated from primary cells or cancer cell lines that self-assemble into simple, spherical cell aggregates.1-3,5,6 Spheroid culturing is relatively simple and can be done with or without extracellular matrix (ECM) support.2,3,6 There are now a number of different spheroid culture models and methods including the hanging-drop method, ultra-low attachment plates, hydrogels, rotary cell cultures, microfluidics systems, and bioprinting.2,3,6
Spheroids are advantageous given their simplicity, shorter culture timeline, and relatively low cost compared to organoids.5 3D spheroid cultures surpass the capability of their 2D culture counterparts particularly in drug screening.2,4 For example, tumor-derived spheroids are comprised of tumor cell types and mimic the physical tumor properties, allowing for a direct study of the tumor microenvironment.2,4,5 Because spheroids are relatively simple, one of their limitations is the lack of polarity and self-organization into higher order tissue structures, so they don’t always represent the in vivo environment.4
Table 1. Comparison of Spheroid vs Organoid 3D Culture Models1,2,4-7
| Spheroids | Organoids | |
|
Cell Source |
Primary cells, cell lines, multicellular mix, or tumor cells and tissues. |
Adult and embryonic stem cells, induced pluripotent stem cells (iPSCs), tumor cells and tissues, or progenitor cells. |
|
Architecture and Morphology |
Typically, a uniform, spherical structure that self-assembles via cell-cell adhesion and aggregation. |
Self-organization of differentiating organ-specific cells into a more complex morphology that recapitulates the organ structure or tissue of origin. |
|
Culture Conditions |
Can be cultured with or without extracellular matrix (ECM), as some cells generate their own during formation, and growth factors. |
Often requires addition of ECM and supplementary growth factors. |
|
Culture Timeline |
Approximately 2-3 days |
21-28 days and longer |
| Maintenance | Difficult to maintain long-term | Long-term viability |
|
Applications |
Study of tumor microenvironment, drug screening, and biomarker discovery. |
Disease and cancer modeling, organ development, drug screening, personalized medicine. |
Organoids: Greater 3D Culture Complexity
In the late 1980s, in vitro cultures originating from lung tissue and neuroblastomas were generated and referred to as organoids.1 Unlike spheroids, organoids are generated from tissue-specific progenitor cells, or stem cells, and require addition of ECM, or basement membrane extract (BME), and growth factors to allow for progenitor cell expansion, differentiation, and self-organization into cultures that recapitulate the organ or tissue of origin.2,4 It can take upwards of 2-3 months to achieve full organoid culture complexity depending on the original tissue source and protocol used.2 To date, a variety of tissues have been used to generate human organoid systems including brain, liver, kidney, stomach, and intestine.1,7 Given their self-renewal ability organoids can be cultured for longer periods of time.1
Organoids have a number of benefits including their recapitulation of human physiology, quick establishment and robustness compared to animal models, genetic modification ability, and patient-specific applications.7 However, despite the advances organoid models have brought to the research community, there are still some limitations, such as protocol standardization, line-to-line heterogeneity from individual genetic differences, difficulty in studying the whole organ, and greater cost than many cell lines.7
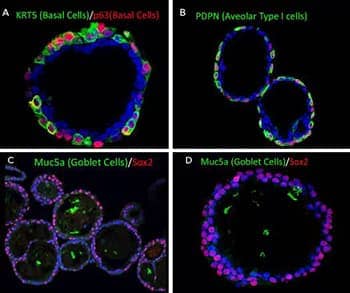
Human Lung Organoids. Adult stem cells were isolated from human lung tissue via biopsy and were embedded in Cultrex UltiMatrix RGF Basement Membrane Extract (Catalog #BME001-05) and cultured for 20-60 days. The lung organoids express various cell type markers of the lung. Lung organoids were stained with A) anti-Cytokeratin 5 (KRT5) (green) and anti-p63/TP73L (red; Catalog # AF1916) to visualize basal cells, B) anti-Podoplanin (PDPN) (green; Catalog # NB600-1015) for alveolar type I cells, and C, D) anti-Muc5ac (green; Catalog # NBP2-15196) to visualize Goblet cells and with anti-Sox2 (red; Catalog # MAB2018). All organoid samples were counterstained DAPI (blue; Catalog # 5748) for nuclei visualization.
Choose Your 3D Culture System
Both spheroid and organoid models are miles ahead of 2D cultures in recapitulating physiological conditions and human pathologies. Additionally, 3D cultures, especially organoids, help bridge the research gap between animal models and humans.7 Each system has its unique set of pros and cons. Organoids are more complex, capable of mimicking the structure and function of an organ, while spheroids are a simpler model system ideal for studying the tumor microenvironment. 1,2,4-7 Undoubtedly, 3D culture techniques will continue to advance. Organoid-on-a-chip, for instance, is an evolving technology that combines organoid culture with microfluidics to create even more physiologically relevant conditions to study organ development, disease, and drug screening.8 The choice between spheroid and organoid models ultimately depends on the research question, the required complexity and project scope, and available resources.

Jennifer Jones, M.S.
Product Marketing Specialist, Antibodies, at Bio-Techne
-
Sakalem ME, De Sibio MT, et al. (2021) Historical evolution of spheroids and organoids, and possibilities of use in life sciences and medicine Biotechnol J. 16:e2000463.
-
Gunti S, Hoke ATK, Vu KP, London NR (2021) Organoid and spheroid tumor models: techniques and applications Cancers (Basel) 13:874.
-
Kim W, Gwon Y, Park S, Kim H, Kim J (2022) Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration Bioact Mater. 19:50-74.
-
Zanoni M, Cortesi M, Zamagni A, et al. (2020) Modeling neoplastic disease with spheroids and organoids J Hematol Oncol. 13:97.
-
Abraham E, Sherman H, Bergeron A. (2020) Spheroids, organoids replacing standard cultures for cell-based assays Gen Eng News.
-
Białkowska K, Komorowski P, et al. (2020) Spheroids as a type of three-dimensional cell cultures-examples of methods of preparation and the most important application Int J Mol Sci. 21:6225.
-
Kim J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology and medicine Nat Rev Mol Cell Biol. 21:571-584.
-
Saorin G, Caligiuri I, Rizzolio F. (2023) Microfluidic organoids-on-a-chip: The future of human models Semin Cell Dev Biol. 144:41-54.