Induced Pluripotent Stem Cells (iPSC)
Pluripotent stem cells (PSC) have the differentiating capacity to serve as the starting point for wide-ranging research into tissue development, drug discovery and toxicology, disease progression, and regenerative medicine.
For cell therapies, induced pluripotent stem cell (iPSC) banks derived from “universal” donors offer the promise of less costly, more rapidly available, and more tightly controlled allogeneic therapies.
At Bio-Techne, our mission is to deliver innovative solutions that enable cell and gene therapies to reach more patients.
On This Page:

About Pluripotent Stem Cells and Cell Therapy
Regenerative medicine and immune cell therapy offer revolutionary promise for the treatment of degenerative diseases, pathogenic genetic defects, tissue damage, and cancer. To produce these cell therapies, somatic cells are recovered from the patient (for autologous therapies) or from immune-compatible donors (for allogeneic therapies) followed by reprogramming to an undifferentiated state by using small molecules. The resulting iPSCs have the capacity to differentiate into multiple cell lineages of the endoderm, mesoderm, and ectoderm germ layers.
An effective cell therapy product requires long term viability and functionality, the ability to home to the correct target tissue, and the ability to evade host immune rejection. Cell and gene therapies that are based on live cells are classified as advanced medicine therapeutic products (ATMPs) which require extensive quality control testing because of their inherent variability.
Listen as Tenielle Ludwig, Director of the WiCell Stem Cell Bank, defines correct use of the terms stemness and pluripotency. These terms are often used interchangeably by researchers and within scientific publications. Dr. Ludwig discusses how consistent use of this nomenclature can positively impact the field of pluripotent stem cell research.
Terminally differentiated somatic cells can be easily recovered from a variety of tissues (e.g. skin fibroblasts or peripheral T cells) and used for generating iPSC.
Somatic Cell Reprogramming to iPSC
iPSC Reprogramming
There are four general groups to categorize an iPSC reprogramming strategy:
- Integrative Reprogramming techniques require the reprogramming factors to be inserted permanently into the host cell genome. This strategy can be further divided into viral and non-viral methods. The pioneering iPSC experiments by Takahashi & Yamanaka (2006) were conducted by integrating four transgenes using Retroviral Vectors.
- Viral reprogramming cells to iPSCs, using lentiviral or retroviral-based transduction methods are the most efficient but have distinct drawbacks for clinical and translational applications
- Non-viral transposons technology such as TcBuster™ is a next-generation solution that avoids some of the pitfalls of virus transductions.
- Non-integrative Reprogramming techniques are the preferred methodology for clinical and translational iPSC generation. They require no genomic integration, and therefore have significantly reduced chance of introducing harmful mutations. Small molecules are widely used in non-integrative reprogramming.

After reprogramming cells to iPSC, confirm their phenotype by the detection of appropriate stemness markers. See Cell Characterization below on this page. Cell Banking Reagents Including Cryopreservation Media, CEPT Cocktail Kit (Catalog # 7991), and ROCK Inhibitors.

Verification of stemness marker expression by multi-color flow cytometry. Human cells were stained using reagents included in the Human/Mouse Pluripotent Stem Cell Multi-Color Flow Cytometry Kit and simultaneously analyzed for SSEA-1, SSEA-4, Oct-3/4, and SOX2. The strong expression of SSEA-4 and Oct-3/4 but not SSEA-1 indicate the undifferentiated status of these cells.
iPSC Expansion and Culture
Careful selection of high quality media components enables robust iPSC expansion with high viability and long term maintenance of the undifferentiated state.
- Application Note: Assessing iPSC Colony Morphology Attributes
- ExCellerate iPSC Expansion Medium
- Growth Factors for iPSC Expansion
- Small Molecules for ES/iPS Cell Expansion
- Cultrex Stem Cell Qualified Basement Membrane Extract (BME)
- Cultrex Basement Membrane Extracts and Extracellular Matrices
- StemXVivo™ Mouse PSC Media Kit
- Stem Cell Media and Supplements
- Mouse Embryonic Fibroblast (MEF) Conditioned Media


Human iPSC cultured in ExCellerate™ iPSC Expansion Medium maintain the expression of stemness markers over long-term culture. These cells express undifferentiated stem cell markers Oct-3/4 (red) and TRA-1-60 (red) along with F-Actin (green) and DAPI (blue) (A). iPSC lines express high levels of Oct-3/4, SSEA-4, SOX2, and no SSEA-1 as assessed by the H/M Pluripotent Stem Cell Multicolor Flow Cytometry Kit (B-C). Undifferentiated stem cell marker expression is >97% across 4 cell lines after more than 45 passages. Graph shows average ± standard deviation.
Gene Engineering for iPSC
Engineer cells to mask them against host immune rejection, improve tissue homing and engraftment, and introduce new functionalities like chimeric antigen receptors (CARs).

Direct visualization of TcBuster-transposed iPSC using the DNAscope™ Assay. Detection was based on a DNAscope probe targeting the TcBuster vector backbone. Wild type iPSC (A), mixed population of TcBuster-transduced iPSC (B), selected clone isolated from the mixed population (C).
Pluripotent Stem Cell Differentiation
Simplify batch bridging during extended cell differentiation processes with reagents strictly qualified for lot-to-lot consistency.
Neuronal Differentiation

Immunocytochemistry of neurons differentiated from iPSC by using the StemXVivo Neural Progenitor Differentiation Kit. Neurons are indicated by Neuron-specific beta-III Tubulin (TUJ1) expression (A), neural progenitors by Pax6 (B), and undifferentiated iPSCs by Oct-3/4 (C). Quantification of images at day 10 and 32 of neuronal differentiation grown on Vitronectin and in ExCellerate iPSC Expansion Medium.
Hepatocyte Differentiation

Immunocytochemistry of iPSC differentiated by using the StemXVivo Hepatocyte Differentiation Kit. Cells were maintained in ExCellerate iPSC Expansion Medium and differentiated to hepatocytes as indicated by Albumin and HNF-4 alpha expression.
Serum-Free and Animal-Free Cell Culture
Increase the consistency of your cell cultures as you approach translational studies for regenerative medicine and cell therapy programs. Adopting these media will
- Reduce variability in media composition
- Simplify compliance with regulatory guidelines
- Simplify comparability testing for raw material changes
Cell Characterization
Cell product qualification is important from start to finish with iPSC programs. Keep a close eye on cell phenotype, secretory profile, culture heterogeneity, and the presence of contaminating particles. After reprogramming, iPSCs should express endogenous pluripotency factors, similar to ESCs. These include both transcription factors, such as Oct4, Sox2, and Nanog, as well as surface markers, like SSEA-4 and TRA-1-60. Interestingly, SSEA-1 is expressed on the surface of mouse, but not human, iPSCs. Learn more about Stem Cell Marker products.
Pluripotent Stem Cell Characterization Kits and Antibodies
- Human Pluripotent Stem Cell Functional Identification Kit
- Human/Mouse PSC Multicolor Flow Kit
- Proteome Profiler™ Human Pluripotent Stem Cell Array Kit
- GloLive Antibodies to Verify Pluripotency in Live Stem Cells
- Human Pluripotent Stem Cell Marker Antibody Panels
Analytical Instrument Platforms and Immunoassays
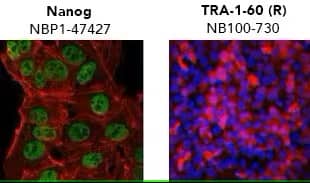
Verification of pluripotency by mmunocytochemistry/ immunofluorescence. (Left) Confocal immunofluorescence analysis of Mouse Anti-Human Nanog Antibody (1E6C4) (Catalog # NBP1-47427) (green). Actin filaments have been labeled with DY-554 phalloidin (red). Nanog staining was confined to the nucleus. (Right) ADLF1 induced pluripotent stem cell line stained with Mouse Anti-Human TRA-1-60 (TRA-1-60) (Catalog # NB100-730) and Anti-Mouse IgG Secondary Antibody (red) and counterstained with DAPI (blue). TRA-1-60 staining was confined to the cell surface.

Single-Cell Western analysis of neuronal differentiation from iPSC. Each dot represents a single cell. iPSC were treated with GMP SB 431542 and GMP Recombinant Human Noggin, followed by terminal differentiation with GMP Recombinant Human FGF, GMP N-2 MAX Media Supplement (100X), and ascorbic acid. Cells were analyzed for Pax6 and Neuron-specific beta-III Tubulin (Tuj). In iPSC, Pax6 was undetectable and 46% of the cells expressed Tuj, while 85% of neurons were Tuj+ Pax6+. See our application note for more details.
- Simple Plex automated ELISAs, 21CFR Part 11-compliant
- Professional Assay Services with RNAscope ISH, GCLP-compliant at ACD
- Micro-Flow Imaging™ particulate analysis, 21CFR Part 11-compliant
- iCE™ Maurice for viral vector analysis, 21CFR Part 11-compliant
iPSC Applications
Disease research often relies on the use of animal models or two-dimensional (2D) in vitro culture systems. Though extremely useful, animal models are limited in their ability to recapitulate complex diseases and accurately model human cellular responses to new drugs and therapies. Traditional in vitro culture systems rely on examining cellular responses in a contrived 2D environment, with cells grown either in a monolayer plastic dish or in suspension surrounded by culture media. Advancements in cell culture techniques to include organoid and 3D cultures that more closely recapitulate in vivo tissue microenvironment, exponentially expand the applications for iPSCs.

Diverse applications of iPSCs. Somatic cells are harvested from patient and reprogrammed into iPSCs. The resulting patient specific iPSCs can then be used in disease modeling and drug screening to generate disease and patient-specific therapies. Additionally, patient-specific iPSCs can be modified to repair genetic mutations. These repaired iPSCs can then be transplanted into the patient to restore tissue functionality.
Drug toxicity screening: new therapies and drugs often fail in human trials due to unforeseen toxicity. To limit this, iPSCs can efficiently be differentiated into organoids to screen target drugs for toxicity. Hepatocytes, neurons, and cardiomyocytes are the three most common tissue for drug toxicity screening.
| Tissue | Selected Markers of Differentiation |
|---|---|
| Hepatocytes | Albumin Transferrin Asialoglycoprotein Receptor (ASGPR1) α-Fetoprotein (AFP) Glutathione-S-transferase P1 (GSTP1) |
|
Neurons |
βIII Tubulin Vimentin GFAP MAP2 Tyrosine Hydroxylase |
|
Cardiomyocytes |
Nkx2.5 Tbx5 Tbx20 Cardiac Troponin I (cTnI ) Cardiac Troponin T (cTnT) Alpha actinin |
Disease modeling: iPSCs allow for direct studying of disease state from the patient’s own cells. Additionally, researchers can directly alter patient cells via gene editing and determine the impact for the individual patient.
- Pancreatic Islet cells for Type 1 Diabetes
- Motor neurons for Amyotrophic Lateral Sclerosis (ALS)
- Intestinal epithelial cells for Inflammatory Bowel Disease (IBD)
- Lung organoids for Cystic Fibrosis
Regenerative Medicine: With the ability to generate disease-free tissues using off-the-shelf or patient-derived cells, iPSCs have a bright future in regenerative medicine.
- Dopaminergic neurons for Parkinson’s Disease (PD)
- Functional cardiac muscle to treat Cardiovascular Disease
- Neurons and glial cells for spinal cord injuries
- Retinal pigmented epithelium (RPE) for Age-related Macular Degeneration (AMD)
During somatic cell reprogramming to iPSCs, there are many genetic and epigenetic changes that occur. With current reprogramming technologies, the epigenetic landscape of the somatic cells is often incompletely or aberrantly modified. This phenomenon, known as epigenetic memory, often biases the iPSCs to differentiate toward their cell of origin.
Resources
Custom Solutions
Custom Solutions
We’re committed to providing optimized solutions to optimize your iPSC workflow. Our custom services team will work with you to deliver reagents and immunoassays that fit your process. Importantly, we have experience developing certified animal-free (AF) reagents as custom projects in cases where AF grade is not otherwise available. We’re experts in the requirements for regulatory compliance as well as custom formulation, vialing, and packaging.
Translational Programs for Cell Therapy
Translational Programs for Cell Therapy
When it’s time to advance your cell therapy product to clinical manufacturing, partner with us for reliable, quality, and/or custom services. We will work with you to provide reproducible production of reagents and assays at clinical scale, with complete documentation. We offer GMP reagents as well as 21 CFR Part 11-compliant analytical instruments for automation and high throughput. We can help you streamline the manufacture of your cell therapies.
Website Resources
- Webinars
- Cell Culture Reagents
- Stem Cells
- Organoid and 3D Cell Culture Products
- Stem Cells Markers Interactive Tool at R&D Systems
- Stem Cell Protocols at R&D Systems
- Streamlining Transition to GMP
- Protocols for Stem Cells at Tocris
- Flow Cytometry Panel Builder
- Cell Expansion and Characterization Instruments
- Cell and Gene Therapy Response Profiling Instruments
- Gene Transfer Assessment Instruments
Literature
- GMP Cytokines and Growth Factors for Therapeutic Manufacturing Brochure
- Accelerating Cell Therapy Discovery & Development With Non-Viral Gene Engineering Article
- Unraveling the Signaling Web in Stem Cells for Regenerative Medicine with Simple Western App Note
- Key Considerations for Cytokine Supplier Selection for Cell Therapies Article
- Stem Cell Research Product Guide
- Basement Membrane Basics Technical Guide at R&D Systems
- Evolution of Cell Culture Models eBook at R&D Systems
- Assessing the Pluripotent Status of Stem Cells Technical Note at R&D Systems
- Differentiation Potential of iPSC Article at R&D Systems
- Next-Generation Analytical Solutions For Cell & Gene Therapy eBook
Background Information
What is an iPSC?
In their initial experiments, Takahashi and Yamanaka isolated mouse fibroblasts and reprogrammed them into cartilage, neural tissue, and columnar epithelium. More recently, iPSCs have been generated from other somatic cells, including peripheral blood mononuclear cells (PBMCs), keratinocytes, and hematopoietic stem cells (HSCs). Less controversial, and equally pluripotent, somatic cells can be reprogrammed by introduction of four transcription factors Oct4, Sox2, Klf4 and c-Myc, often referred to as “Yamanaka Factors.” The addition of two transcription factors, LIN-28 and Nanog, has been shown to increase reprogramming efficiency. After reprogramming to a “de-differentiated” state, iPSCs can generate virtually any cell type.
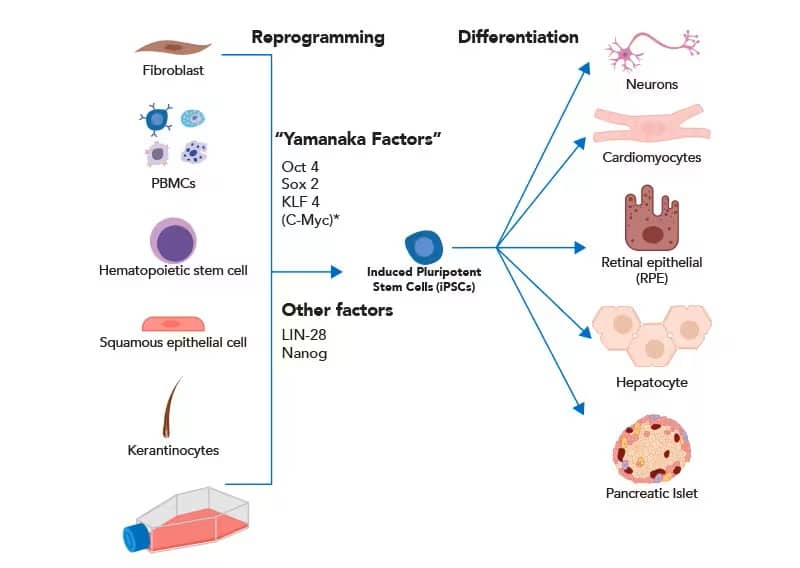
Somatic cells can be cultured and reprogrammed into iPSCs using canonical pluripotency transcription factors. iPSCs are differentiated into various tissues for basic research and translational studies. *Though c-Myc is an essential transcription factor for iPSC reprogramming, high oncogenic potential makes it a poor factor to integrate for translational applications.
Mesenchymal Stem Cells (MSC)
Does StemXVivo™ Mesenchymal Stem Cell Expansion Media need addition of serum before use?
The StemXVivo Mesenchymal Stem Cell Expansion Media does not need addition of serum. Fetal bovine serum is included in the product, which is a complete medium and ready to use. The media may be supplemented with cytokine or growth factors for your desired cell culture application.
How long can mesenchymal stem cells (MSCs) be cultured in StemXVivo Mesenchymal Stem Cell Expansion Media?
MSCs can be grown to 80-90% confluency and subsequently subcultured using the protocol provided in the product datasheet. Researchers should establish the number of passages that is acceptable for their work. MSCs are sensitive to passages and, if subcultured too many times, may start losing their MSC characteristics. Our MSC Functional Identification Kits can be used for validation of MSC mulitpotency.
Does StemXVivo Mesenchymal Stem Cell Expansion Media contain phenol red?
Yes StemXVivo Mesenchymal Stem Cell Expansion Media contains phenol red.
Is the appearance of crystals in StemXVivo Xeno-free Human MSC Expansion Media normal?
Yes. The crystals are a calcium-containing precipitate and may form, to a limited degree, in this media. A limited amount of crystals will not impact MSC expansion. Avoiding multiple freeze-thaw cycles of the medium is helpful in reducing the amount of crystals.
Does the Human Mesenchymal Stem Cell Functional Identification Kit include the adipogenic, osteogenic, and chondrogenic kit supplements?
Yes, the StemXVivo Human Adipogenic Supplement, StemXVivo Human Osteogenic Supplement, and StemXVivo Human Chondrogenic Supplement are the same as Part #s 390415, 390416, and 390417, respectively, in the Human Mesenchymal Stem Cell Functional Identification Kit.
Is it important to use medium without ribonucleosides and deoxyribonucleosides for growing mesenchymal stem cells?
We have not compared, in a side-by-side experiment, medium with and without ribonucleosides and deoxyribonucleosides for growing mesenchymal stem cells. There is literature to support the fact that using medium without ribonucleosides and deoxyribonucleosides is beneficial for growing mesenchymal stem cells.
Can the Human Mesenchymal Stem Cell Functional Identification Kit be used with non-human primate mesenchymal stem cells?
It is likely that the antibodies included in the kit are cross-reactive to other primates. The supplements included in the kit are not intended to be species-specific. However, the kit has not been tested with primate mesenchymal stem cells.
For the Human Mesenchymal Stem Cell Functional Identification Kit, how can induction of differentiation be monitored?
For adipogenic differentiation, the appearance of vacuoles in cells after 5-7 days is a sign of differentiation and can be monitored by microscopic examination of the cells. For osteogenic differentiation, the beginning of cell detachment after about 14 days is a sign of differentiation. Cell detachment should be monitored in this case. For chondrogenic differentiation, there isn't an exact marker to look for other than fixing and staining the frozen pellet between differentiation days 14 - 21. The exact choice of time may take some empirical testing.
Will the Human or Mouse Mesenchymal Stem Cell Multi-Color Flow Kit work for identification of rat MSCs?
The Human Mesenchymal Stem Cell Multi-Color Flow Kit or Mouse Mesenchymal Stem Cell Multi-Color Flow Kit will not work for identification of rat mesenchymal stem cells.
Are there any experimental tips/hints for successful chondrogenic differentiation of mesenchymal stem cells?
The following tips/hints are useful for chondrogenic differentiation: a) The mesenchymal stem cells (MSCs) should not be from a late passage (passage 8 or less), b) If using the Human Mesenchymal Stem Cell Functional Identification Kit or the StemXVivo Chondrogenic Supplement, use the starting MSC cell number that is indicated in the protocol, c) Early during chondrogenic differentiation a pellet should form. As differentiation progresses, the pellet will grow and take up a ball-like appearance. d) The pellet should not attach to the tube, therefore care should be taken to not dislodge it while changing media.
Can human mesenchymal stem cells (MSCs) be used with the StemXVivo Cardiomyocyte Differentiation Kit?
This kit is unlikely to work with human MSCs as these cells are at a different point in development and are expected to need different signals.
Neural Cells
What is the difference between N-2 Plus Media Supplement and N-2 MAX Media Supplement?
The N-2 Plus Media Supplement contains bovine insulin whereas N-2 MAX Media Supplement contains recombinant human insulin. All other media components and concentrations in N-2 Plus and N-2 MAX are the same. These two products perform comparably in side-by-side testing. Due to a limited supply of bovine insulin, the products are priced differently.
Does N-2 MAX Media Supplement contain any components that are calcium salts?
No.
Does N-2 MAX Media Supplement contain any proteins that are produced using Baculovirus?
No.
Does the N-2 MAX Media Supplement contain any animal-derived components other than human transferrin?
No. The N-2 MAX Media Supplement does not contain any animal-derived components other than Human Transferrin. GMP N-2 MAX Media Supplement is an animal-free supplement.
Should NPCs be maintained on plates coated with Cultrex™ RGF BME?
Yes. During NPC maintenance, cells should be cultured on plates coated with Cultrex RGF BME. Coating a plate with Cultrex Poly-L-Lysine (10 µg/mL) followed by Cultrex Laminin (20 µg/mL) is another option.
Can Human/Mouse/Rat Neural Lineage Functional Identification Kit be used with both embryonic and adult neural progenitor cells?
The Human/Mouse/Rat Neural Lineage Functional Identification Kit has been tested with embryonic neural progenitor cells. The kit should also work with adult neural progenitor cells, as adult cells have similar growth conditions.
Can the Human/Mouse Dopaminergic Neuron Differentiation Kit work with a starting population of neural progenitor cells instead of mouse pluripotent stem cells?
The kit may work with a starting population of neural progenitor cells, but we have not tested this. If starting with neural progenitor cells we recommend starting at Stage 5 of the Dopaminergic Neuron Differentiation Kit protocol. The starting cell density would also have to be optimized.
How many passages can the neural progenitor cells (NPCs), obtained on Day 7 of differentiation, go through before being used in an experiment/application?
Following differentiation NPCs can be passaged approximately five times. However, it is ideal to cryopreserve the NPCs following the 7-8 day differentiation and save the frozen cells for future experiments.
What are the recommendations for using the neural progenitor cells (NPCs) for differentiation into astrocytes and oligodendrocytes?
One recommendation is to maintain the NPCs at a cell density of about 50,000-100,000 cells/cm2. The second is to allow the NPCs to go through 2-4 passages before differentiating into astrocytes and oligodendrocytes as older NPCs show better differentiation into these neurons.
Cardiomyocytes
How many days does it take to generate cardiomyocytes using the StemXVivo Cardiomyocyte Differentiation Kit?
Beating cells can be seen as early as Day 10-13 of differentiation. Beating then becomes more widespread throughout the following week.
When should I switch from the Cardiomyocyte Differentiation Kit to the Cardiomyocyte Maintanence Media Supplement?
The switch from the Cardiomyocyte Differentiation Kit to the Cardiomyocyte Maintanence Media Supplement can be made any time after beating is initiated or upon the completion of the reagents provided in the kit.
Hepatocytes
At what time point after starting hepatocyte differentiation do cells reach the definitive endoderm stage?
The cells are differentiated to the definitive endoderm stage by around day 4 from differentiation initiation. A schematic of the differentiation progression can be viewed in this scientific poster.
Luminex is a registered trademark of Luminex Corporation.
