By Hunter Martinez
The paradigm shifting view of the immune system being leveraged to target cancer has led to numerous therapeutic breakthroughs. One major cell group responsible for this revelation is a T cell. T cells functioning in fighting cancer can become tired or “exhausted”. T cell exhaustion is a state of cellular dysfunction resulting from repeated stimulation and chronic exposure to antigen. T cell differentiation to exhaustion is phenotypically demarcated by expression of inhibitory molecules like PD-1 and reduced secretion of pro-inflammatory cytokines such as IFN-γ. However, contributions from cytokines, cell intrinsic metabolic pathways, and the metabolic environment to T cell exhaustion remain unclear. In a recent publication, Scharping et al. untangle the relationship between the metabolic environment of the tumor and T cell exhaustion. Specifically, whether the metabolic environment directly contributes to terminal differentiation and exhaustion of T cells or if differentiation towards T cell exhaustion is deterministic of the cells metabolic state.
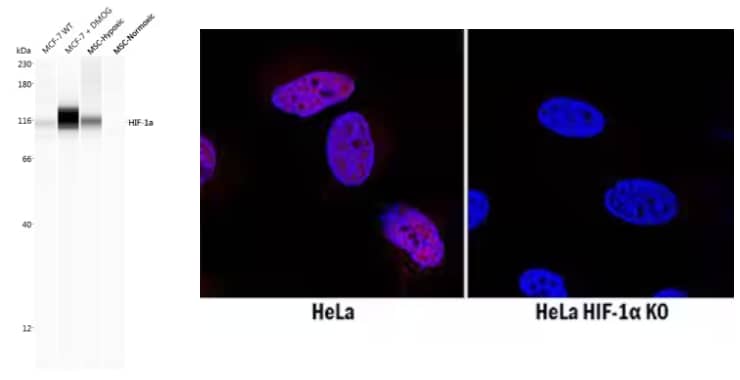
|
(Left) Biological Strategies Validated. Simple Western lane view analysis of lysates from MCF-7 breast cancer cell line with (+) and without (WT) DMOG treatment, and BioSpherix MSCs in either hypoxic or normoxic conditions. Lysates were probed with Rabbit Anti-HIF-1 alpha Polyclonal Antibody (Catalog # NB100-134) followed by Anti-Rabbit Secondary HRP Antibody (Catalog # 042-206, ProteinSimple). A band for HIF-1 alpha is shown at ~116 kDa, with increased expression in lysates treated with DMOG and in hypoxic conditions. This experiment was conducted under standard assay conditions using the 12-230 kDa separation system. (Right) Genetic Strategies Validated. Comparison of HIF-1 alpha expression in immersion-fixed WT HeLa cell line, treated with desferoxamin (DFO), versus HIF-1 alpha knockout (KO) HeLa cell line using Mouse Anti-HIF-1 alpha Monoclonal Antibody (Catalog # MAB1536), followed by staining with NorthernLights™ 557-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # NL007) (red). HIF-1 alpha antibody staining was localized to the nuclei. Nuclei were counterstained with DAPI (blue). |
Hypoxia Reinforces T cell Exhaustion
To address the question of the involvement of the metabolic environment, the authors focused on studying the effects of hypoxia, a metabolic state involving insufficient oxygen levels commonly found in tumors. First, the researchers examined if terminally exhausted tumor infiltrating lymphocytes (TILs) experience more hypoxia than other subsets. Administration of a hypoxia tracer into the tumors of mice revealed the phenotypically exhausted cells exhibited the highest degree of hypoxia-inducible factor 1-alpha (HIF1α) expression and hypoxia tracer staining. These results demonstrate terminally exhausted T cells experience elevated hypoxia compared to less exhausted subsets. Next, to mimic the in vivo tumor environment, CD8+ T cells were continuously stimulated with antibodies against the T cell receptor (TCR) under high or low oxygen levels. CD8+ T cells with persistent stimulation under low oxygen (hypoxia) expressed more coinhibitory molecules (e.g. LAG-3, TIGIT) and had limited ability to simultaneously secrete multiple cytokines (also known as polyfunctional cytokine secretion), all hallmarks of a terminally exhausted cell. Taken together, these results indicate the metabolic environment, specifically hypoxia, alters cellular differentiation and contributes to T cell exhaustion. Surprisingly, HIF1α was dispensable for achieving the exhausted phenotype, as deletion still resulted in lost polyfunctionality after continuous stimulation under hypoxia.
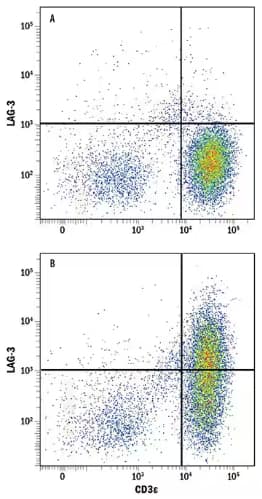
Flow Cytometry analysis showing human peripheral blood mononuclear cells (PBMCs) either (A) untreated or (B) treated with Phytohemagglutinin (PHA), which stimulates T cell activation, and stained using Goat Anti-Human LAG-3 Polyclonal Antibody (Catalog # AF2319) followed by staining with Allophycocyanin (APC)-conjugated Anti-Goat IgG Secondary Antibody (Catalog # F0108) and Phycoerythrin (PE)-conjugated Mouse Anti-Human CD3 epsilon Monoclonal Antibody (Catalog # FAB100P). Quadrants were set based on staining with Goat IgG Isotype Control Antibody (Catalog # AB-108-C).
The Role of ROS in T cell Exhaustion
Persistent stimulation of T cells alone or hypoxia alone did not cause reduced metabolic pathway activity, so the team hypothesized these two factors may prevent metabolic reprogramming through transcriptional means. PGC1α is a transcriptional co-activator previously shown to be involved in metabolic reprogramming through mitochondrial biogenesis and antioxidant activity and to prevent T cell exhaustion. Armed with this knowledge, they found that Blimp-1 represses PGC1α. To more fully understand the repression of PGC1α and the mechanism underlying T cell dysfunction caused by stimulation under hypoxia, the researchers overexpressed PGC1α. PGC1α-overexpressing T cells displayed increased mitochondrial metabolism, upregulation of antioxidant enzymes, and regulation of reactive oxygen species (ROS), while preventing the expression of genes associated with terminal exhaustion. These data suggest the ability to manage ROS production is critical to prevent differentiation towards exhaustion. Independent of TCR stimulation, hypoxia induces persistent mitochondrial ROS which increases phosphotyrosine signaling, induces Nur77 signaling (a downstream component of TCR signaling), and increases nuclear factor of activated T cells (NFAT) localization. Together the findings show that in combination with chronic TCR signaling, hypoxia reinforces the T cell exhaustion program through mitochondrial dysfunction and accumulation of ROS.
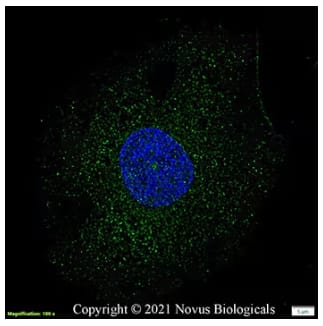
Immunocytochemistry/Immunofluorescence analysis of fixed and permeabilized HeLa cells incubated with Rabbit Anti-PGC1 alpha Antibody (Catalog # NBP1-04676) and detected with an Anti-Rabbit Dylight 488 Antibody (green). Nuclei were counterstained with DAPI (blue).
In summary, the authors demonstrate a role for the metabolic environment contributing to T cell exhaustion and terminal differentiation. Specifically, the inability to undergo metabolic reprogramming and mitigate ROS production due to a hypoxic environment inhibits the T cells’ ability to produce cytokines and results in decreased cytolytic killing. It will be exciting to see if future T cell therapeutics for the treatment of solid tumors include ways to directly modulate the tumor microenvironment or, perhaps, directly engineer the T cells to cope with metabolic stress and ROS generation.

Hunter Martinez
Stanford University School of Medicine
Hunter is a PhD graduate student studying the role of the extracellular matrix in T cell activation and function.
Research in Focus
Scharping NE, Rivadeneira DB, Menk AV, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021; 22(2):205-215. https://doi.org/10.1038/s41590-020-00834-9