By Jennifer Jones, M.S.
Biosimilar Antibodies: Imitation Meets Innovation
In the ever-evolving medical landscape, a new class of pharmaceuticals has emerged as a game-changer, transforming the way we approach treatment options: Biosimilars. Biosimilars are biologics, such as antibodies and proteins, that demonstrate therapeutic equivalency to the original approved drug, also known as the reference product. The Food and Drug Administration (FDA) defines a biosimilar as having “no clinically meaningful differences in terms of safety, purity, and potency (safety and effectiveness) from an existing FDA-approved biologic”.1 Moreover, they are equivalent to the therapeutic drug in terms of functionality, pharmacokinetics, and immunogenicity.2 As of September 2023, the FDA had approved 43 biosimilars, including many monoclonal antibodies (mAbs).1,3 Examples of FDA-approved monoclonal biosimilar antibodies, their target molecule, and therapeutic area are listed in Table 1.
Table 1. Examples of Biosimilar Antibodies and Their Therapeutic Areas 1,3
| Biosimilar | Target Molecule | Therapeutic Areas |
|
TNF-α |
Crohn’s disease, Psoriasis, Psoriatic arthritis, Rheumatoid arthritis (RA), Ulcerative colitis |
|
|
VEGF |
Colorectal cancer, Non-small cell lung cancer (NSCLC), Renal cell carcinoma |
|
|
CD20 |
Chronic lymphocytic leukemia (CLL), Non-Hodgkin’s lymphoma (NHL), Rheumatoid arthritis (RA) |
|
|
ErbB2/Her2 |
Breast Cancer |
The Role of Biosimilar Antibodies in Targeted Cancer Therapy
Direct tumor elimination is the common goal of many cancer immunotherapy mAbs. Developed to target both solid tumors and hematological cancers, these mAbs function to:
- Block receptor-ligand binding
- Induce cell lysis
- Tag tumor cells for opsonization4
Biosimilars in this context are valuable counterparts to the original therapeutic antibodies, providing equivalent effectiveness in targeting tumors and enhancing immune responses. They are crucial players in the quest for more accessible treatment options.
One such group of ligand-blocking biosimilars target members of the EGFR family, including ErbB2/Her2.5,6 Her2 is overexpressed in almost 30% of breast cancer cases, making it a prime target for biosimilar antibodies like Trastuzumab, which works by blocking ligand binding and disrupting receptor function by preventing Her2 homodimerization.
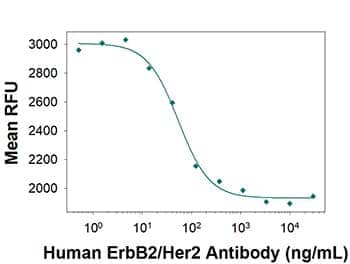
Cancer Cell Proliferation Inhibition by ErbB2/Her2 (Trastuzumab Biosimilar) Antibody. Neutralization assay showing inhibition of SK-BR-3 human breast cancer cell line proliferation by Human Anti-Human ErbB2/Her2 (Research Grade Trastuzumab Biosimilar) Monoclonal Antibody (Catalog # MAB9589) as measured by Resazurin (Catalog # AR002). The median effective dose for this effect is typically 15-75 ng/mL.
Biosimilar mAbs also target hematopoietic differentiation antigens like CD30/TNFRSF8, CD52, and CD20. For example, Rituximab targets CD20, a B-cell maturation marker, and is used to treat non-Hodgkin’s lymphoma (NHL) and other hematological cancers. Rituximab-bound B-cells are eliminated by cell lysis engaging the Fc region of the antibody and FcγR receptors on immune cells. This activation leads to the release of pro-inflammatory compounds and the demise of targeted tumor cells.
Immune checkpoint Inhibitors
Another breakthrough approach for anticancer mAb-based therapy targets a special class of proteins called immune checkpoint proteins. Immune checkpoint proteins, such as CTLA-4 and PD-1,6 are critical mediators of any immune response. Immune checkpoint inhibitor mAbs target these proteins and function to boost the body's immune response against cancer. One of the most well studied immune checkpoints is the PD-1/PD-L1 receptor-ligand interaction. PD-1 is expressed on the surface of T cells and functions to inhibit T cell activation, while PD-L1 is often overexpressed on target tumor cells. The anti-PD-1 and anti-PD-L1 antibodies Nivolumab (Opdivo®) and Atezolizumab (Tencentriq®), respectively, are inhibitors that work to restore T cell function and counteract this negative regulation. To further investigate this important immune checkpoint, Bio-Techne recently added Pembrolizumab (anti-PD-1) to our biosimilar antibody portfolio.
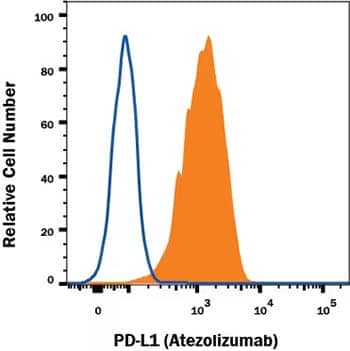
Flow Cytometry Analysis Showing Detection of PD-L1/B7-H1 antibody in MDA-MB-231 human breast adenocarcinoma cell line. MDA-MB-231 human breast adenocarcinoma cell line was stained with Human Anti-Human PD-L1/B7-H1 (Atezolizumab Biosimilar) Monoclonal Antibody (Catalog # MAB10348, filled histogram) or irrelevant antibody (open histogram) followed by APC-conjugated Goat Anti-Human IgG Secondary Antibody (Catalog # F0135).
Conclusions and the Future of Biosimilars in the Fight Against Cancer
Biosimilar antibodies are becoming crucial tools in the fight against cancer, offering new hope for patients battling this deadly disease. These biologically equivalent counterparts not only expand access to cutting-edge therapies but also offer cost-effective solutions that can revolutionize healthcare. As research continues to advance, we can anticipate an even brighter outlook for biosimilar antibodies and more innovative treatments on the horizon.

Jennifer Jones, M.S.
Product Marketing Specialist, Antibodies, at Bio-Techne
-
U.S. Food and Drug Administration (2023) Biosimilars. https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/biosim….
-
Nabhan C, Parsad S, Mato AR et al. (2018) Biosimilars in oncology in the United States: a review. JAMA Oncol 4:241-247.
-
Generics and Biosimilars Initiative (2023) Biosimilars approved in the US.
-
Liu J, Pandya P, Afshar S. (2021) Therapeutic advances in oncology. Int J Mol Sci 22:2008.
-
Weiner LM, Surana R, Wang S (2010) Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 10:317-327.
-
Scott AM, Allison JP, Wolchok JD (2012) Monoclonal antibodies in cancer therapy. Cancer Immun. 12:14.