Heparan Sulfate 3-O-Sulfotransferase 4/HS3ST4 Products
Heparan sulfate is a highly sulfated polysaccharide that can be found on the cell surface and within the extracellular matrix. It is typically covalently attached to the protein core of proteoglycans, such as syndecans and glypicans. Heparin, on the other hand, can be considered as a highly sulfated version of heparan sulfate that is predominantly found in mast cells. Both heparin and heparan sulfate contain disaccharide repeats of uronic acid and N-acetylglucosamine and are modified by the same sulfotransferases. The uronic acid residues can be sulfated at the 2-O position by heparan sulfate 2-O sulfotransferase (HS2ST). The N-acetylglucosamine residues can be sulfated at the N, 3-O, and 6-O positions by N-deacetylase/N-sulfotransferases (NDSTs), heparan sulfate 3-O sulfotransferases (HS3STs) and heparan sulfate 6-O sulfotransferases (HS6STs) respectively. There are seven HS3STs in the human genome. HS3ST4 and HS3ST2 are brain specific and may participate in HS-dependent neurobiologic events. HS3ST4 can generate tetrasulfated heparan sulfate disaccharide, the most highly sulfated sugar found in biological samples, and may have a role in assisting HSV-1 entry and spread. HS3ST4 is a Golgi resident type II membrane protein and has the longest proline rich stem region among all HS3STs. The enzyme activity was assayed using an SDS-PAGE based method.
4 results for "Heparan Sulfate 3-O-Sulfotransferase 4/HS3ST4" in Products
4 results for "Heparan Sulfate 3-O-Sulfotransferase 4/HS3ST4" in Products
Heparan Sulfate 3-O-Sulfotransferase 4/HS3ST4 Products
Heparan sulfate is a highly sulfated polysaccharide that can be found on the cell surface and within the extracellular matrix. It is typically covalently attached to the protein core of proteoglycans, such as syndecans and glypicans. Heparin, on the other hand, can be considered as a highly sulfated version of heparan sulfate that is predominantly found in mast cells. Both heparin and heparan sulfate contain disaccharide repeats of uronic acid and N-acetylglucosamine and are modified by the same sulfotransferases. The uronic acid residues can be sulfated at the 2-O position by heparan sulfate 2-O sulfotransferase (HS2ST). The N-acetylglucosamine residues can be sulfated at the N, 3-O, and 6-O positions by N-deacetylase/N-sulfotransferases (NDSTs), heparan sulfate 3-O sulfotransferases (HS3STs) and heparan sulfate 6-O sulfotransferases (HS6STs) respectively. There are seven HS3STs in the human genome. HS3ST4 and HS3ST2 are brain specific and may participate in HS-dependent neurobiologic events. HS3ST4 can generate tetrasulfated heparan sulfate disaccharide, the most highly sulfated sugar found in biological samples, and may have a role in assisting HSV-1 entry and spread. HS3ST4 is a Golgi resident type II membrane protein and has the longest proline rich stem region among all HS3STs. The enzyme activity was assayed using an SDS-PAGE based method.
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG2a Monoclonal Clone #712011 |
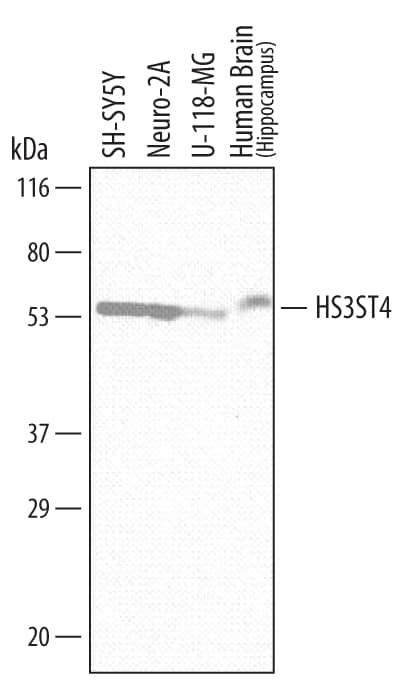
| Applications: | WB |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #712010 |
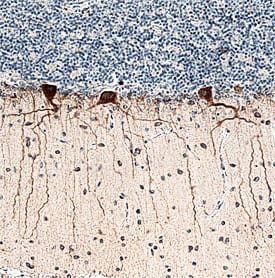
| Applications: | IHC, IP |
| Reactivity: | Human, Mouse |
| Details: | Sheep IgG Polyclonal |
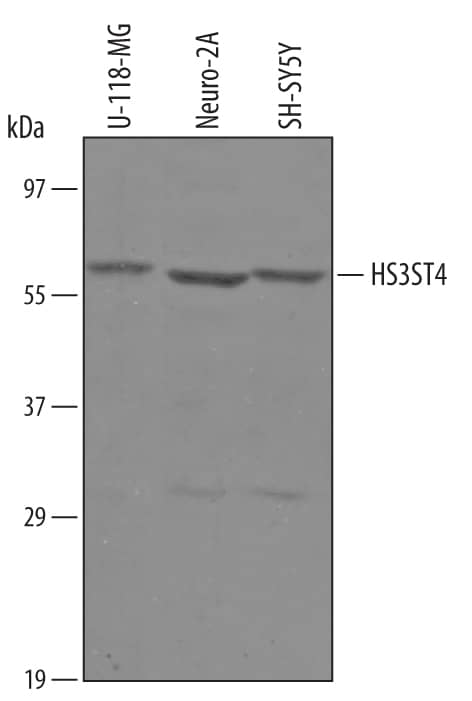
| Applications: | WB, IHC |
| Source: | CHO |
| Accession #: | NP_006031 |
| Applications: | EnzAct |