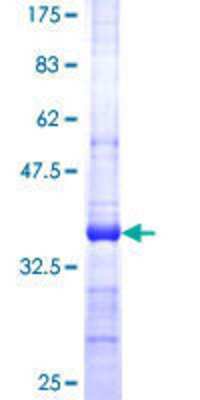
SerpinB9: Proteins and Enzymes
SerpinB9 is a serine proteinase inhibitor of the ovalbumin like B clade of serpins. The mouse orthologue to PI9 is SPI-6. SerpinB9 was first discovered in human bone marrow in a search for serpins similar to SerpinB6. SerpinB9 was identified in lymphocytes, especially natural killer cells and cytotoxic T lymphocytes, cells which also produce and store the apoptosis-inducing enzyme Granzyme B. PI9 was shown to be a potent inhibitor of Granzyme B, working at picamolar efficiencies. PI9 was also shown to inhibit caspase 1, the interleukin 1 converting enzyme, thus blocking IL1 223; production, and decreasing inflammatory processes. Most leukemia cells and many tumor cells produce PI9, leading to speculation that PI9 may help protect tumor cells from destruction by NK and CTL cells. PI9 is also found in placenta, lung, kidney, mast cells, and many tissues and cell lines. In kidney transplant, PI9 is sharply elevated, and in the liver PI9 has been shown to be upregulated by a downstream estrogen promoter. SerpinB9 inhibits Granzyme B, and is cleaved by Granzyme M, as a down regulatory step. SerpinB9 also inhibits the bacterial proteinase subtilisin A, and this may be a protective agent against infections. Neutrophil elastase is also a target of SerpinB9. Like SerpinB6 and SerpinB8, SerpinB9 lacks a signal sequence, and is found mainly in the cytoplasm and nucleus, although it can be detected outside of cells and in serum. The original SerpinB9 sequence described was 379 amino acids in length, with predicted mass of 42.4 kDa and pI of 5.51.
Show More
2 results for "SerpinB9 Proteins and Enzymes" in Products
2 results for "SerpinB9 Proteins and Enzymes" in Products
SerpinB9: Proteins and Enzymes
SerpinB9 is a serine proteinase inhibitor of the ovalbumin like B clade of serpins. The mouse orthologue to PI9 is SPI-6. SerpinB9 was first discovered in human bone marrow in a search for serpins similar to SerpinB6. SerpinB9 was identified in lymphocytes, especially natural killer cells and cytotoxic T lymphocytes, cells which also produce and store the apoptosis-inducing enzyme Granzyme B. PI9 was shown to be a potent inhibitor of Granzyme B, working at picamolar efficiencies. PI9 was also shown to inhibit caspase 1, the interleukin 1 converting enzyme, thus blocking IL1 223; production, and decreasing inflammatory processes. Most leukemia cells and many tumor cells produce PI9, leading to speculation that PI9 may help protect tumor cells from destruction by NK and CTL cells. PI9 is also found in placenta, lung, kidney, mast cells, and many tissues and cell lines. In kidney transplant, PI9 is sharply elevated, and in the liver PI9 has been shown to be upregulated by a downstream estrogen promoter. SerpinB9 inhibits Granzyme B, and is cleaved by Granzyme M, as a down regulatory step. SerpinB9 also inhibits the bacterial proteinase subtilisin A, and this may be a protective agent against infections. Neutrophil elastase is also a target of SerpinB9. Like SerpinB6 and SerpinB8, SerpinB9 lacks a signal sequence, and is found mainly in the cytoplasm and nucleus, although it can be detected outside of cells and in serum. The original SerpinB9 sequence described was 379 amino acids in length, with predicted mass of 42.4 kDa and pI of 5.51.
Show More
| Applications: | WB, ELISA, MA, AP |
| Applications: | AC |