Janelia Fluor® Dyes for Super-Resolution Microscopy
Developed at the Janelia Research Campus, Janelia Fluor® dyes are exceptionally bright and highly photostable. They are well suited for super-resolution techniques including STED, PALM, and dSTORM.
Janelia Fluor dyes are supplied with a range of reactive groups for biomolecular conjugation, including:
- Amine-Reactive
- Click-Reactive (azide and tetrazine)
- Free acid
- Sulfhydryl-Reactive (Maleimide)
Conjugation protocols for Janelia Fluor dyes are available on tocris.com.
All amine reactive dyes can be converted to relevant substrates for use in self-labeling tag systems such as HaloTag® and SNAP-tag®.
All images kindly provided by Professor. C. Soeller, University of Exeter; images taken by Alex Clowsley and Anna Meletiou.
SNAP-tag is a trademark of New England BioLabs, Inc and HaloTag is a trademark of Promega Corporation.
Janelia Fluor is a registered trademark of Howard Hughes Medical Institute.
Photoactivatable Janelia Fluor® Dyes
Photoactivatable Janelia Fluor® Dyes
Photoactivatable Janelia Fluor® dyes are a favorable alternative to photoactivatable proteins for PALM microscopy. PA Janelia Fluor® 646, SE (Cat. No. 6150) and PA Janelia Fluor® 549, SE (Cat. No. 6149) can be used on both lived and fixed cells and can be multiplexed to perform two-colour sptPALM in live cells. Individually these dyes are suitable for single molecule tracking and PALM and sptPALM.
Spontaneously Blinking Janelia Fluor® Dyes
Spontaneously Blinking Janelia Fluor® Dyes
Spontaneously Blinking Janelia Fluor® Dyes are ideal for single-molecule localization microscopy (SMLM).
These Janelia Fluor® dyes have a duty cycle optimized for super-resolution microscopy experiments.
Antibodies
Antibodies
Bio-Techne brands R&D Systems and Novus Biologicals, offer 780,000 antibodies which have been validated in over 25 species for more than 15 applications.
Luke Lavis: Bright Ideas in Fluorescence Imaging
In this episode of the Back of the Napkin podcast, discover how the work of Luke Lavis, at the Janelia Research Campus, has pushed the boundaries of fluorescence imaging. His development of Janelia Fluor® dyes has significantly improved live-cell and super-resolution imaging capabilities.
This podcast reveals the intricate balance between fundamental research, application in drug discovery, and the collaborative culture at Janelia, emphasizing the importance of mentorship and interdisciplinary interactions.
Super-Resolution Microscopy Types and Tips
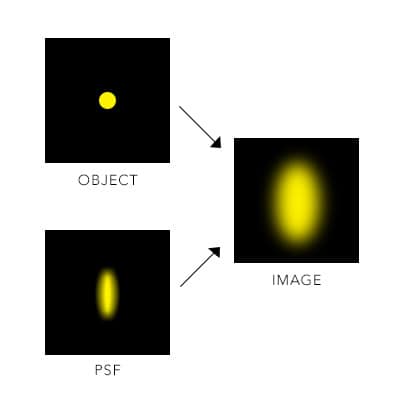
Figure 1: Point spread function. Diffraction of light through media and a lens causes a blur, which limits the resolution of the image being acquired to approximately 200 nm.
Super-resolution microscopy is the umbrella term for a group of techniques that break the optical diffraction limit, producing higher resolution images compared to conventional light microscopy. In conventional light microscopy, the resolution of images is limited to approximately 200 nm. This is because when two structures are closer than this diffraction limit, they will blur into one. The blur size is known as the point spread function (PSF). Figure 1.
To circumvent the limit imposed by the point-spread function, super-resolution microscopy techniques broadly work by either reducing the size of the “effective” point spread function or preventing the fluorescent labels in a sample from being too close in either space or time. Super-resolution microscopy images can achieve approximately 20 times the resolution compared to conventional light microscopy, allowing researchers to investigate biological structures and processes at the nanoscale and to perform single molecule localization and single particle tracking.
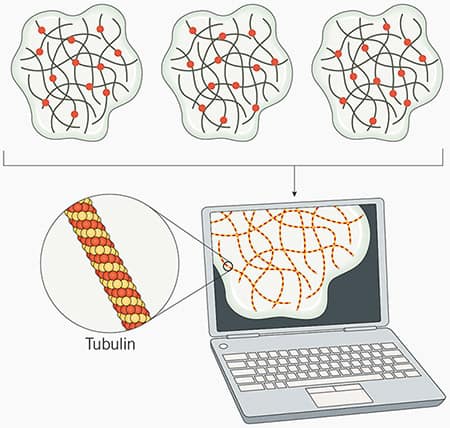
Figure 2: STORM and dSTORM microscopy principles. In STORM microscopy, fluorophores are stochastically excited over time. Subsets of individual fluorophores randomly “blink”, switching between an “off” or “dark” state, to an “on” or “emission” state.. This process is repeated numerous times until most or all the fluorophores have been imaged. Each fluorophore’s precise location is determined by capturing the coordinates for each set of emitted photons. The data is then combined by specialized software to produce a high-resolution image of the sample (in this example tubulin is imaged). For dSTORM, the principals are the same as STORM microscopy, but dSTORM uses fluorophores that are inherently capable of stochastic activation are used, so no activator dye is required.
What is Stochastic Optical Reconstruction Microscopy or STORM microscopy?
Stochastic Optical Reconstruction Microscopy or STORM microscopy stochastically images subsets of photoswitchable fluorophores over a period of time, allowing spatial resolution of individual fluorophores even in a densely labeled sample. STORM microscopy is the most popular super resolution microscopy method and has produced images with resolution as high as 5 nm.
STORM microscopy relies on stochastic activation of dye molecules (fluorophores) that switch between on and off states (often referred to as ‘blinking’). When imaged over a period of time, the combination of sequential activation/inactivation and time-resolved mapping makes it is possible to precisely locate the position of the fluorophores and construct a detailed image of the structures being imaged. Due to the nature of STORM microscopy process, the fluorophores used need to be photostable, very bright and be capable of photoswitching.
Imaged fluorophores must be separated by at least the light diffraction limit (~200 nm), so that only one fluorophore is activated within the diffraction limit in one instance, otherwise a blur will occur and the resolution of the image will be reduced.
dSTORM Microscopy: how does it differ from STORM Microscopy
There are two types of STORM microscopy, the first referred to simply as STORM, and the second direct STORM or dSTORM. STORM microscopy involves a pair of activator-reporter dyes, where the activator dye induces photoswitching and the reporter dye emits the photons. dSTORM utilizes fluorophores that are inherently capable of stochastic activation (e.g. HM Janelia Fluor® 526, SE, Cat. No. 7312) and so does not require an activator dye.
Much like STORM, photoactivated localization microscopy (PALM) and fluorescence photoactivation localization microscopy (FPALM), rely on capturing the emission of a few fluorophores at a time. In PALM this is achieved by using photoactivatable fluorophores that reside in a non-fluorescent ‘dark’ state until laser activated. The fluorophores are activated stochastically in small numbers. When computationally reconstructed, an image is produced with very high resolution.
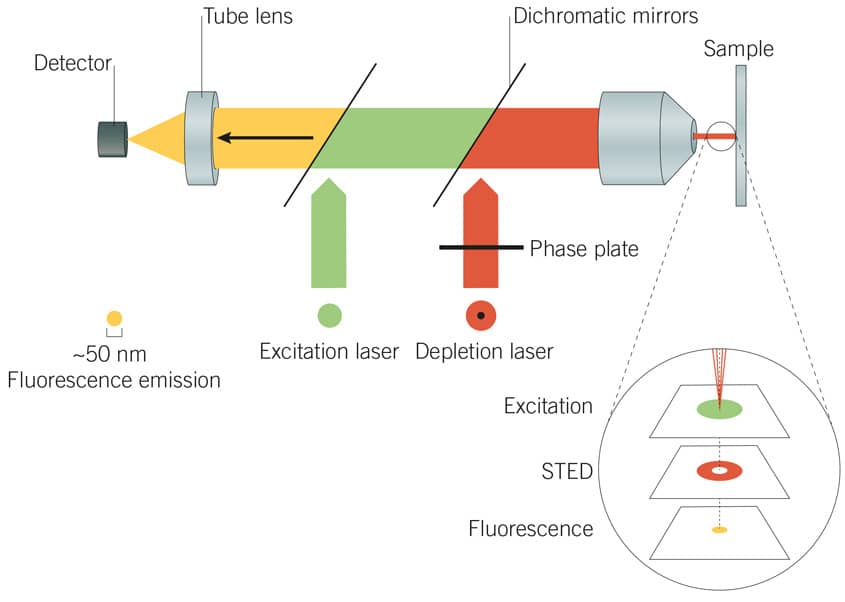
Figure 3: Principles of STED microscopy. A STED laser suppresses photon emissions around the periphery of the excitation laser focus to effectively reduce the point-spread function, producing a high-resolution image.
How does stimulated emission depletion (STED) microscopy work?
Stimulated emission depletion (STED) microscopy employs two lasers to achieve resolution improvement via spatially patterned excitation. The excitation laser is used in tandem with a STED laser, which selectively suppresses or ‘depletes’ photon emissions around the periphery of the excitation laser focus. This effectively achieves suppression of a ring of fluorophores around the excitation laser focus (the “depletion donut”), restricting fluorescent excitation to the small centre-point of the donut. The point spread function is thus effectively reduced beyond the natural diffraction limit of light.
STED microscopy can achieve image resolution of up to 30 nm. For the best results with STED, dye excitation and depletion wavelengths should align with the specifications of the instrument being used.
Other types of super-resolution microscopy that use patterned excitation include saturated structured-illumination microscopy (SSIM) and reversible saturable optically linear fluorescence transitions (RESOLFTs).
Fluorophores for super-resolution microscopy should ideally be very bright; highly photostable (exhibiting minimal photobleaching especially in commonly used thiol-containing buffers); capable of stochastic photoswitching with a low duty cycle (meaning the period spent in the “on” emitting state is less than that spent in the “off” non-emitting dark state) .
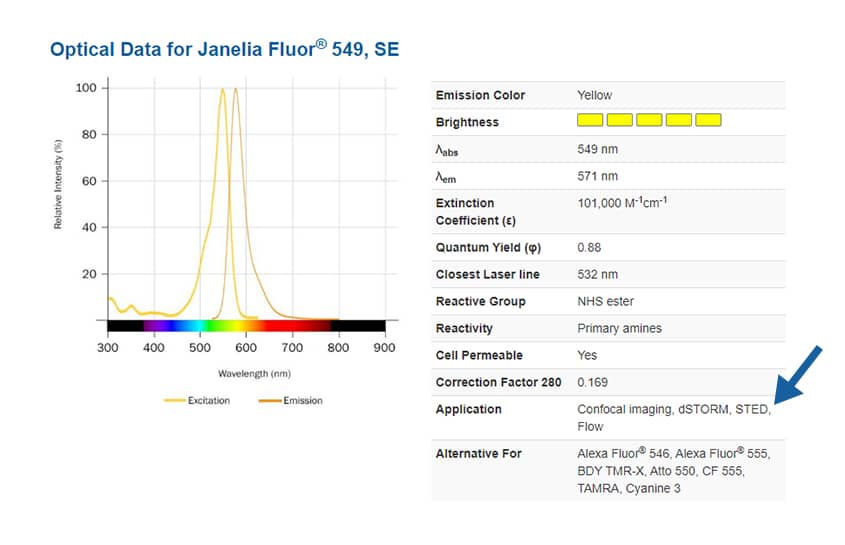
To find suitable fluorophores for your super-resolution microscopy experiment, see the application notes on each dye product page, where you will also find absorbance and emission spectra, extinction coefficient, quantum yield and closest laser line.
Specific buffers are often required to allow fluorescent dyes to efficiently photoswitch. Two of the most widely used buffers contain a basic buffering system and either mercaptoethylamine (MEA) or β-mercaptoethanol (BME). MEA is favored when using xanthene-based dyes such as Janelia Fluor® dyes, and cyanine-based dyes favor BME and TCEP buffers.
For example buffer recipes read:
Dempsey et al (2011) Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat Methods 8 1027 PMID 22056676
Vaughan et al (2013) Phosphine quenching of cyanine dyes as a versatile tool for fluorescence microscopy. J. Am. Chem. Soc. 135 1197 PMID 23311875
Fluorescent Dyes and Probes Brochure
This product guide provides a comprehensive list and background to the use of fluorescent dyes, probes, stains, and reagents, and contains information on our:
- Fluorescent Dyes, including Janella Fluor® Dyes
- Fluorescent Probes for Advanced Microscopy and Live Cell Imaging
- Fluorescent Probes to Support In Vivo, Deep Tissue, and Bioluminescence Imaging
- Fluorescent Probes and Reagents for Organoids and 3D Cell Culture Imaging
- Aptamer-based RNA Imaging Reagents
- Fluorescent Probes for Imaging Bacteria
- TSA Reagents for Enhancing IHC, ICC & FISH Signals, including TSA Vivid™ Fluorophore Kits
- Tissue Clearing Kits and Reagents
-
Zheng et al. (2019) Rational design of fluorogenic and spontaneously blinking labels for super-resolution imaging. ACS Cent.Sci. 5:1602. PMID: 31572787.
-
Grimm et al. (2017) A general method to fine-tune fluorophores for live-cell and in vivo imaging. Nat. Methods 14:987. PMID: 28869757.
-
Legant et al. (2016) High-density three-dimensional localization microscopy across large volumes. Nat.Methods 13:359. PMID: 26950745.
-
Grimm et al. (2020) A general method to optimize and functionalize red-shifted rhodamine dyes. Nat.Methods 17:815. PMID: 32719532.
-
Grimm et al. (2016) Bright photoactivatable fluorophores for single-molecule imaging. Nat.Methods 13:985. PMID: 27776112.
-
Long et al. (2020) Super resolution microscopy and deep learning identify Zika virus reorganization of the endoplasmic reticulum. Sci.Rep. 10:20937. PMID: 33262363.






