|
|
Strategy for N-glycan labeling.
Glycan labeling is achieved through direct incorporation of Cy3-conjugated Neu5Ac (Cy3-Neu5Ac) (A) or replacement of natural sialic acids with Cy3-Neu5Ac (B). In both strategies, Cy3-Neu5Ac is introduced via recombinant ST6Gal1. In strategy B, samples are first desialylated with recombinant C. perfringens Neuraminidase (Neu) that specifically removes alpha 2-3 and alpha 2-6 linked sialic acids. Alternative fluorophore conjugated Neu5Ac donor substrates and sialyltransferases may be used for the labeling.
|
|
|
Specific detection of N- and O-glycans on different glycoproteins.
Recombinant Human CEACAM-7 Protein (9010-CM), Recombinant Human CEACAM-8/CD66b Protein (9639-CM), Recombinant Human MUC-1 Fc Chimera Protein (10332-MU), and Recombinant Human CA125/MUC16 Protein (5609-MU) were labeled on N-glycan (N) by ST6Gal1 with Cy3-Neu5Ac according to the protocol and on O-Glycan (O) by Recombinant Human ST3GAL2 Protein (7275-GT) with Cy5-Neu5Ac and then separated on 4-20% gradient SDS-PAGE gel. Only N-glycans were detected on CEACAM-7 and CEACAM-8, and only O-glycans were detected on MUC-1, both N- and O-glycans were detected on MUC16. The left part is the TCE image of the gel and the right part is the fluorescent image.
|
|
|
N-glycan and O-glycan specific labeling of recombinant human ACE-2.
N-glycan labeled recombinant human (rh) ACE-2 was prepared according to the provided protocol and O-glycan labeled rhACE-2 was prepared in the same way except that ST6Gal1 was replaced with O-glycan specific ST3Gal2. The labeled samples were then treated with 10-fold serial dilution of PNGase F starting with 100 ng using the PNGase F N-glycan Releasing Kit (EA006). The digestions were separated on 17% SDS-PAGE gel. The upper part is the fluorescent image and the lower part is the TCE image of the gel. While all labeled N-glycans could be released by PNGase F N-glycan Releasing Kit, no labeled O-glycans were released by PNGase F, demonstrating the specific labeling on N- and O-glycans by ST6Gal1 and ST3Gal2, respectively.
|
|
|
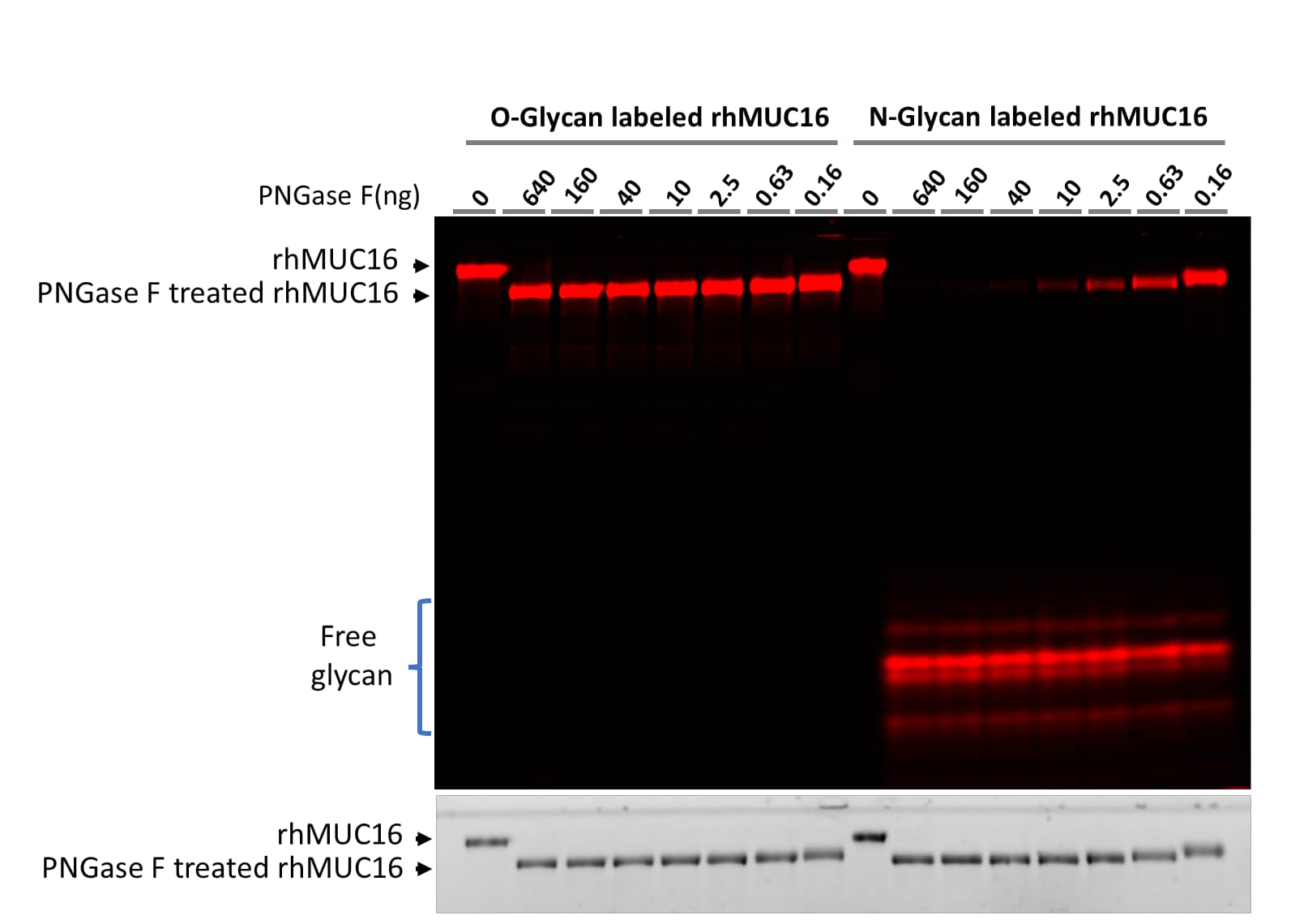
N-glycan and O-glycan specific labeling of Recombinant Human MUC16.
Recombinant Human CA125/MUC16 Protein (5609-MU) was prepared according to the above protocol except that Cy3 was replaced with Cy5 in CA125/MUC16 and ST6Gal1 was replaced with ST3Gal2 for O-glycan labeling. The labeled samples were treated with 4-fold serial dilution of PNGase F starting with 640 ng using the PNGase F N-glycan Releasing Kit (EA006). The digestions were separated on 17% SDS-PAGE gel. The upper part is the fluorescent image and the lower part is the TCE image of the gel. While all labeled N-glycans could released by PNGase F N-glycan Releasing Kit, no labeled O-glycans were released by PNGase F, demonstrating the specific labeling on N- and O-glycans by and ST6Gal1 and ST3Gal2, respectively.
|
|
|
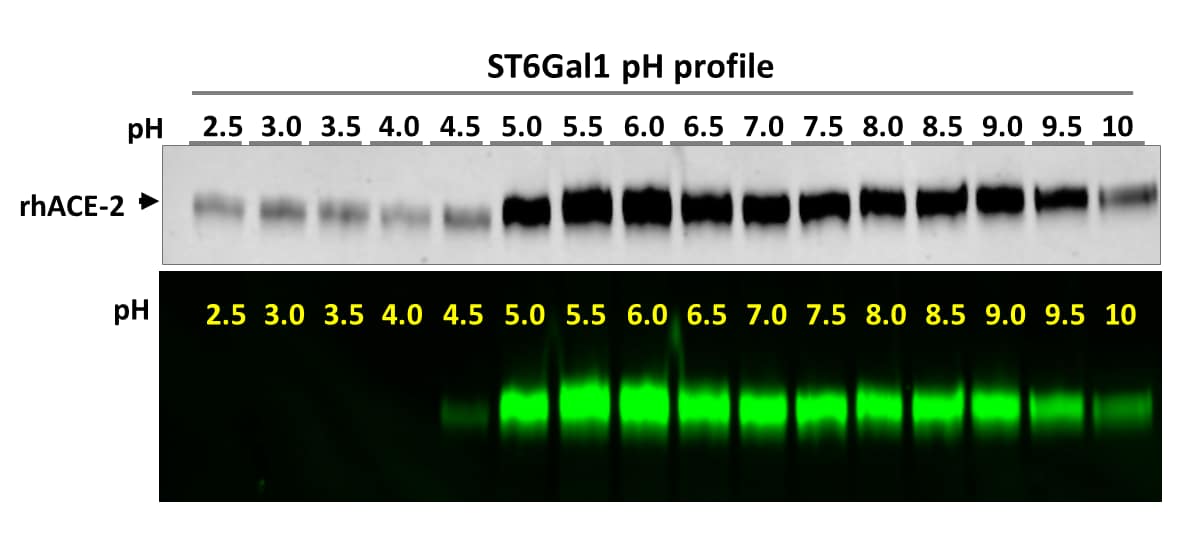
Determining the optimal pH for ST6Gal1 labeling.
Optimal pH determination of a sialyltransferase was an extremely difficult task in the past. Using the N-glycan Labeling and Detection Kit (Catalog # EA007), this task becomes rather easy. In this figure, Recombinant Human ACE-2 was labeled by ST6Gal1 at different pH following the provided protocol except with different buffers. The labeled reactions were then separated on a 4-20% gradient SDS-PAGE. Both TCE protein image and fluorescent image are shown. Incorporation of Cy3 to rhACE-2 resulted in intensified band absorption in TCE images. While ST6Gal1 is active from pH 4.5-10, its optimal pH ranges from 5-9.
|
|
|
Effect of neuraminidase treatment on N-glycan labeling.
Recombinant Human ACE-2 Protein (933-ZN) and Recombinant Human CA125/MUC16 Protein (5609-MU), without or with Neuraminidase (Neu) treatment, were labeled by ST6Gal1, then digested with PNGase F N-glycan Releasing Kit (EA006) and finally separated on 17% SDS-PAGE gel. Both the protein image stained with TCE (upper panel) and the fluorescent image (lower panel) are shown. The freed glycans are visible in both images. While Neuraminidase treatment slightly increased labeling on ACE-2, it greatly enhanced the labeling on MUC16, indicating that the majority N-glycans on MUC16 were initially sialylated. This data demonstrates that the kit is also a good tool to evaluate the sialylation levels on target proteins.
|
|
|
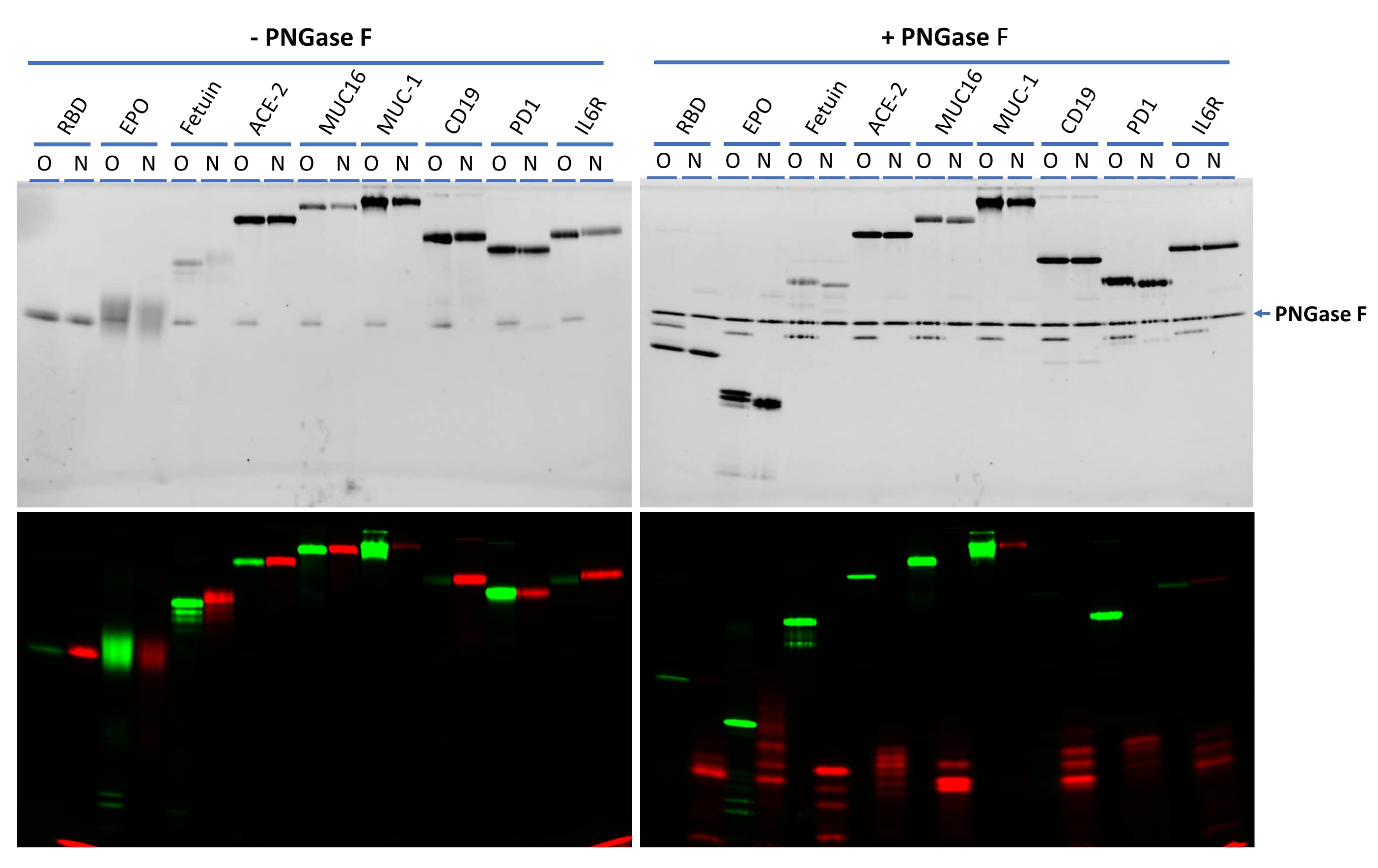
Effect of neuraminidase treatment on N-glycan labeling.
A collection of glycoproteins were labeled on O-glycans with Cy3-Neu5Ac (green) by ST3Gal2 and N-glycans with Cy5-Neu5Ac (red) by ST6Gal1. The left side are gel images of labeled samples without PNGase F treatment. The right side are gel images of the samples after treatment with PNGase F N-glycan Releasing Kit (EA006). While MUC-1 mainly contains O-glycans and CD19 mainly contains N-glycans, the other proteins contain both N- and O-glycans at different levels. All labeled N-glycans (in red) except that on MUC-1 could be released by PNGase F N-glycan Releasing Kit, further confirming the specific labeling. The nature of the labeling on MUC-1 by ST6Gal1 is not clear. All samples were separated on 17% SDS-PAGE gel. Recombinant SARS-CoV-2 Spike RBD His-tag Protein (10500-CV), Recombinant Human Erythropoietin/EPO Protein, Recombinant Human ACE-2 Protein (933-ZN), Recombinant Human CA125/MUC16 Protein (5609-MU), Recombinant Human MUC-1 Fc Chimera Protein (10332-MU), Recombinant Human CD19 Fc Chimera Protein (9269-CD), Recombinant Human PD-1 Fc Chimera Protein (1086-PD) and Recombinant Human IL-6R alpha Protein (227-SR) were from Bio-Techne®. Fetal bovine fetuin was from Sigma Aldrich.
|