Exosome Isolation and Detection
It is well established that extracellular vesicles (EVs), including exosomes, are important mediators of intercellular communication in health and disease. However, the specific contribution of EVs compared to other extracellular messengers can be difficult to elucidate. The first step of determining the precise role of EVs is to isolate them from biofluids or cell culture media.
There are many methods available to isolate EVs and each researcher must determine which method is best suited to their research question. Is the goal to determine a specific biomarker for cancer carried by EVs? In this case, the isolation method may focus more on quantity, rather than specificity, of EVs. Is the goal to determine what cargo is carried by EVs secreted from a particular cell type? Then perhaps, a more targeted isolation approach is required.
How Do You Isolate Exosomes?
The chosen method of isolation determines the yield and relative “specificity” of an EV preparation. Traditionally, exosome isolation has been a time consuming and arduous task with many centrifugation steps at extremely high speeds. Other methods have been developed that have a simpler workflow and a faster turnaround time but may result in isolation of both exosomes and microvesicles. There are advantages and challenges associated with each technique, and each researcher must decide based on their individual system and ultimate goals which technique is optimal for them.
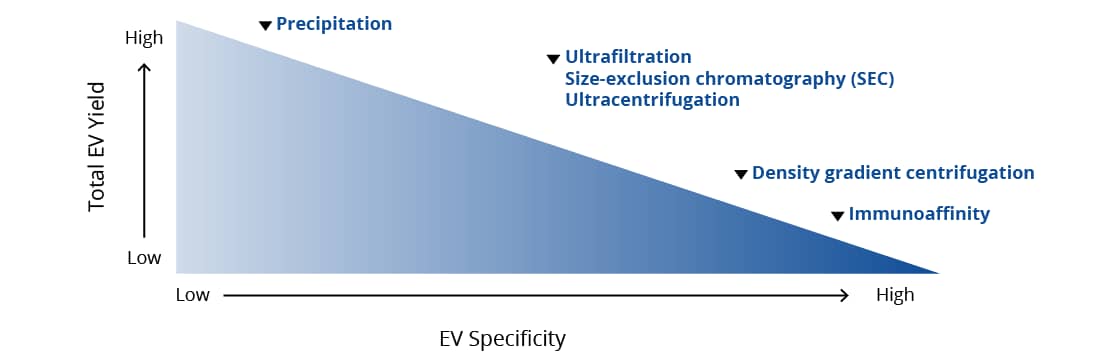
In addition to the challenges associated with each technique, individual biofluids present complications based on their composition. For example, biological fluids such as plasma and serum are extremely complex and have higher viscosity than cell culture medium. Often, isolation of EVs from these biofluids requires faster and longer ultracentrifugation steps than when cell culture media is the source. Additionally, urine is an extremely useful source of EVs from the kidneys, bladder, and prostate, but the abundance of Tamm-Horsfall protein (THP) in urine can obscure detection of subtle, but important, changes in EV composition and protein expression. It is highly recommended to remove THP with a salt precipitation step prior to EV isolation.
| Biofluid | Possible Contaminants/Co-isolated with EVs | Primary EV Source |
|---|---|---|
| Plasma |
|
|
| Urine |
|
|
| Cerebrospinal Fluid (CSF) |
|
|
Common Methods of EV Isolation
Precipitation
Yield: Intermediate Specificity: Intermediate (Varies)
Of the common techniques used to isolate EVs, precipitation offers one of the fastest turnarounds and most straightforward workflows. Briefly, samples containing EVs are incubated with a buffer that changes their solubility or sedimentation rate. One common precipitation buffer uses polyethylene glycol (PEG), a hydrophilic polymer. By incubating EVs with PEG, it decreases the solubility of EVs in solution and, using a very gentle centrifugation step, causes accumulation of EVs at the bottom of the tube. Some other common methods of precipitation include sodium acetate and organic solvents. Precipitation results in a large volume of isolated EVs with no specificity for EV subtype or specific proteins.
| Advantages | Disadvantages | |
|---|---|---|
| Precipitation |
|
|
| Product | Specifications |
|---|---|
| EV Precipitation Solution (Blood) | For precipitation of EVs from small volumes (100-250 µL) of plasma and serum |
| EV Precipitation Solution (Blood) | For precipitation of EVs from up to 5 mL of cell culture supernatant |
| EV Precipitation Solution (Urine) | For precipitation of EVs from up to 20 mL of urine |
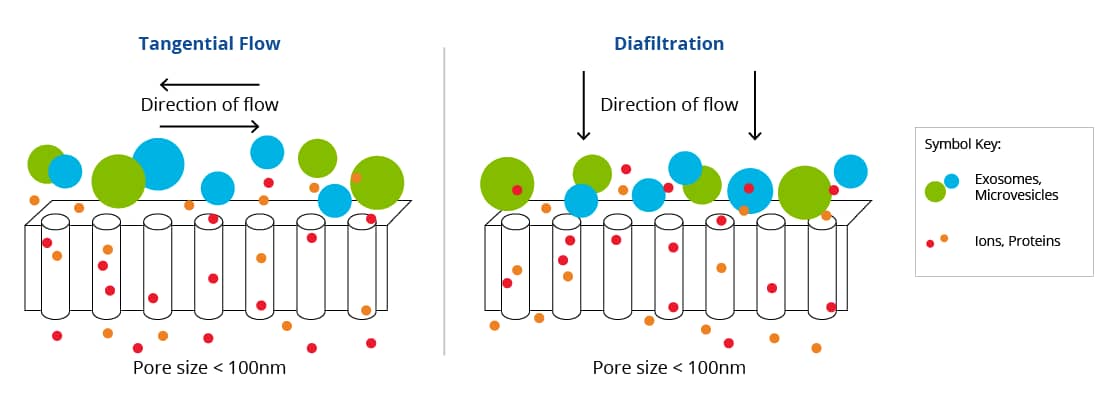
Ultrafiltration is the process the fluid passing over a semi-permeable membrane, allowing some substances to pass through while others are retained. In the case of EV isolation, ultrafiltration involves passing your sample over a porous membrane filter between 0.08-0.1 µm in diameter. Due to the semi-permeable nature of the membrane, ultrafiltration will concentrate the sample, as well as separate molecules based on size. This technique is especially useful for isolating EVs from dilute biofluids, such as urine, as well as cell culture media. Broadly, there are two types of ultrafiltration commonly used for EV isolation: tangential flow filtration (TFF) and diafiltration. TFF passes the liquid horizontally (tangentially) over the pores of the membrane allowing particles that are smaller than the membrane pores to filter through, while larger particles are retained in the chamber. Diafiltration involves pushing the mixture directly up or down through the membrane pores.
| Advantages | Disadvantages | |
|---|---|---|
| Ultrafiltration Diafiltration and Tangential Flow Filtration (TFF) |
|
|
| Product | Specifications |
|---|---|
| Tangential Flow Filter (TFF)- EV Concentrator | For concentration of dilute matrices like urine and cell culture medium prior to EV isolation. |
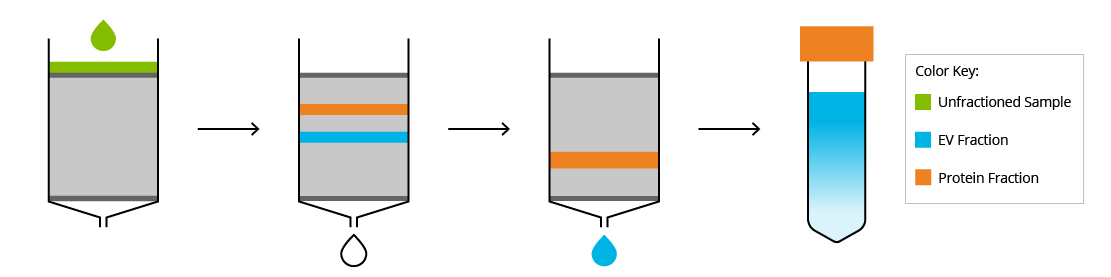
As indicated by the name, size-exclusion chromatography (SEC) separates macromolecules based on their size. To accomplish this, the sample is passed through a porous column and molecules are isolated in fractions from the eluate. The size of the pores can vary depending on the size of the desired product. For isolation of EVs from proteins and lipoproteins, pore sizes can vary from ~40-80 nm. As the sample passes through the column, molecules smaller than the pore size will get trapped in the pores, slowing them down, while larger molecules will pass through. This results in the larger molecules, such as EVs, eluting from the column in earlier fractions, while smaller molecules, such as proteins and protein aggregates, are eluted in later fractions. To optimally use SEC for dilute matrices, such as urine or cell culture media, first these samples must be concentrated by an ultracentrifugation or ultrafiltration step.
| Advantages | Disadvantages | |
|---|---|---|
| Size-exclusion Chromatography |
|
|
| Product | Specifications |
|---|---|
| EV SEC Columns | To isolate EVs from ~2 mL plasma or concentrated conditioned media and urine. |
| EV MaxiSEC Columns | To isolate EVs from ~20 mL concentrated conditioned media |
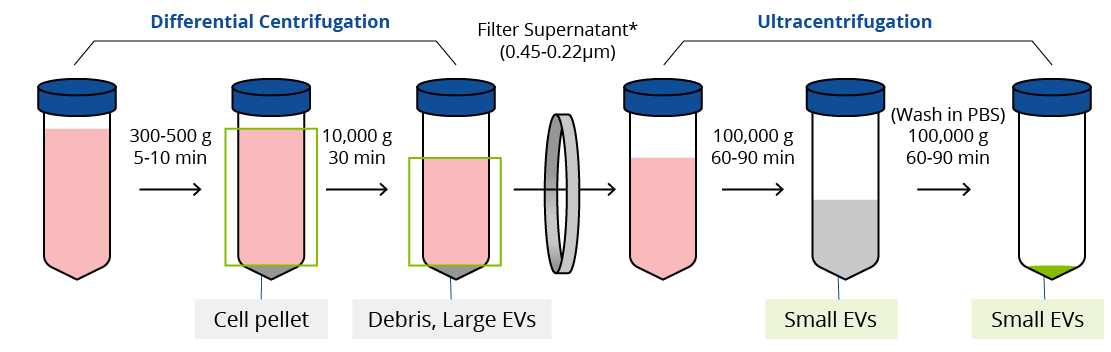
Considered the gold standard for EV isolation, ultracentrifugation with differential centrifugation relies on vesicle size and density. Briefly, samples are taken through several cycles of centrifugation to clear the supernatant of cells, cellular debris, and larger vesicles such as apoptotic bodies. Then the sample is subjected to either one or two rounds of ultracentrifugation (100,000-200,000 g). Some protocols suggest an additional filtration step using a 0.45-0.22 µm filter prior to ultracentrifugation.
| Advantages | Disadvantages | |
|---|---|---|
| Ultracentrifugation |
|
|
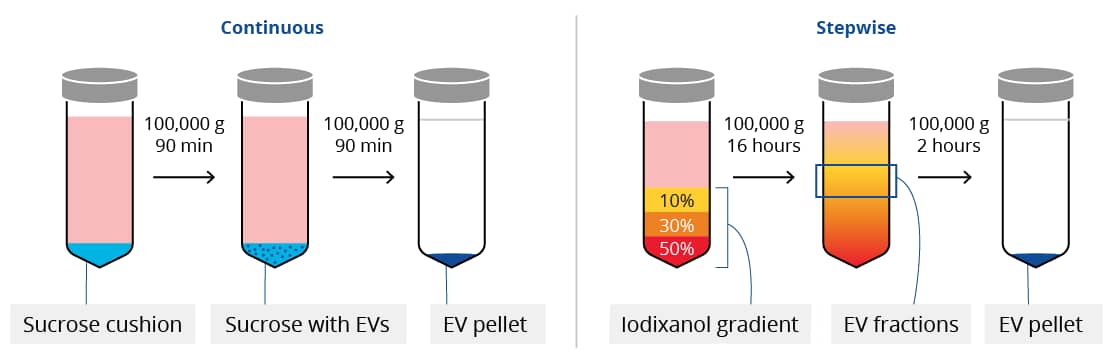
To further improve upon the specificity of EV isolation, many researchers use density gradient centrifugation. There are two general types of density gradient centrifugation for EV isolation: continuous and stepwise gradients. Often called a “cushion”, continuous density gradient centrifugation involves underlaying a sucrose gradient of a singular density to the sample prior to ultracentrifugation. For stepwise, or discrete, density gradient centrifugation, sample is overlayed on layers of iodixanol with different densities.
| Advantages | Disadvantages | |
|---|---|---|
| Density Gradient Centrifugation (Sucrose or Idoixanol) |
|
|
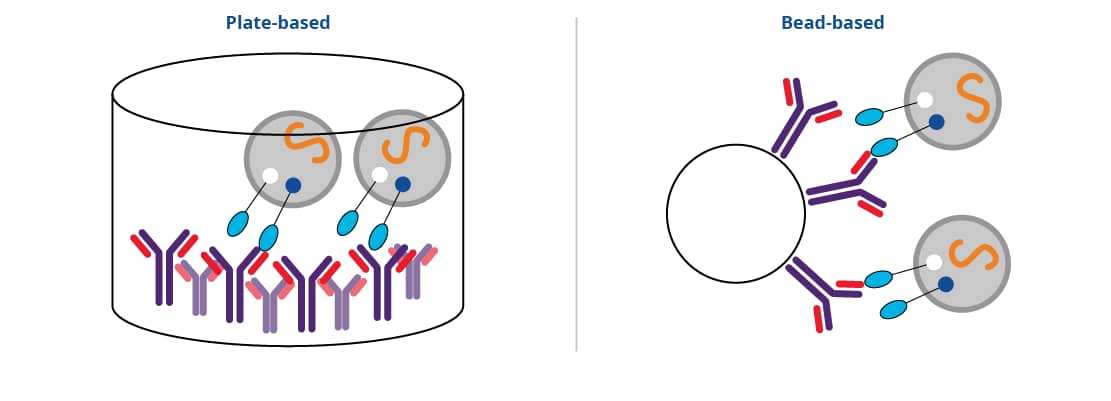
As more cell-type and disease-specific proteins are identified, more researchers are turning to immunoaffinity capture techniques for EV isolation. This technique relies on the antigen-antibody interaction. Antibodies directed at target of interest (eg CD9, CD63) are coated either in a 96-well plate or conjugated to a bead. Sample is applied directly into coated well, or into sample with coated beads, and EVs are allowed to bind. In a final step, EVs can be eluted from the antibody and used in downstream functional and phenotypic analysis. This method of isolation is highly specific, resulting in extremely pure EV populations.
| Advantages | Disadvantages | |
|---|---|---|
| Immunoaffinity capture |
|
|
| Product | Specifications |
|---|---|
| CD9 Immunobeads for Exosome Isolation | Coated with anti-human CD9 for isolation of exosomes from plasma, serum, and concentrated urine. |
| CD63 Immunobeads for Exosome Isolation | Coated with anti-human CD63 for isolation of exosomes from conditioned cell media. |
How Do You Detect Exosomes?
There are many methods available to confirm the presence of EVs after isolation. No matter the chosen method of detection, it is important to compare the isolated EV preparation to a known sample containing EVs of interest. Bio-Techne offers lyophilized exosome standards from a range of cell types, disease states, and biological fluids to serve as a positive control in detection assays.
| Method | Advantages | Disadvantages |
|---|---|---|
| Electron Microscopy |
|
|
| Western Blot |
|
|
| Simple Western |
|
|
| ELISA |
|
|
| Flow Cytometry |
|
|
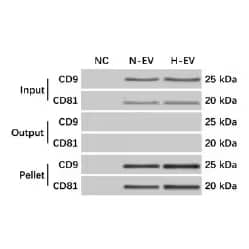
Western blot of mesenchymal stem cell (MSC)-EV precipitation from MSCs culture media was verified by western blot. Blots were probed with Mouse Anti-Human CD9 (5G6) (NBP2–22187) and Mouse Anti-Human CD81 (1D6) (NB100-65805). Input, media prior to ultracentrifugation. Output, supernatant after ultracentrifugation. Pellet, EV pellet after ultra-centrifugation. NC, negative control. N-EV, EV from naïve MSCs. H-EV, EV from hypoxia challenged MSCs. Image from Ren W, et al. (2019) Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J Exp Clin Cancer Res 38 62. Licensed under CC license.
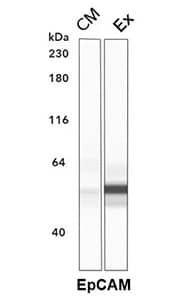
Simple Western lane view of conditioned media (CM) and exosomal fraction (Ex) of human colorectal adenocarcinoma cell line HT-29. Exosomal fraction was isolated using precipitation method. Using polyclonal Goat Anti-Human EpCAM/TROP1(AF960) a strong signal is detected in the Ex, but not CM, lane.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:. https://doi.org/10.1080/20013078.2018.1535750.
Doyle L, Wang M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019;8:727. https://doi.org/10.3390/cells8070727.
Crescitelli R, Lässer C, Lötvall J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc 2021;16:1548–80. https://doi.org/10.1038/s41596-020-00466-1.
Konoshenko MY, Lekchnov EA, Vlassov A V., Laktionov PP. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int 2018. https://doi.org/10.1155/2018/8545347.
Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Théry C. SnapShot: Extracellular Vesicles. Cell 2020;182:262-262.e1. https://doi.org/10.1016/j.cell.2020.04.054.