Exosome Marker Antibodies
The International Society for Extracellular Vesicles (ISEV) has defined the minimum requirements for the reproducible and accurate identification and investigation of exosomes in human health and disease. These requirements include exosome marker antibodies that should be used to discriminate extracellular vesicles (EVs) from contaminating components in culture media or biological fluids.
The presence of exosomes should be confirmed by detection of at least one transmembrane or GPI-anchored protein and one cytosolic lipid or membrane associated protein. To assess the degree of contamination of an exosome preparation with cellular components, assays should also include targets that rarely associate with EVs like albumin, ApoA1, Calnexin, or ribosomal protein S6/RPS6.
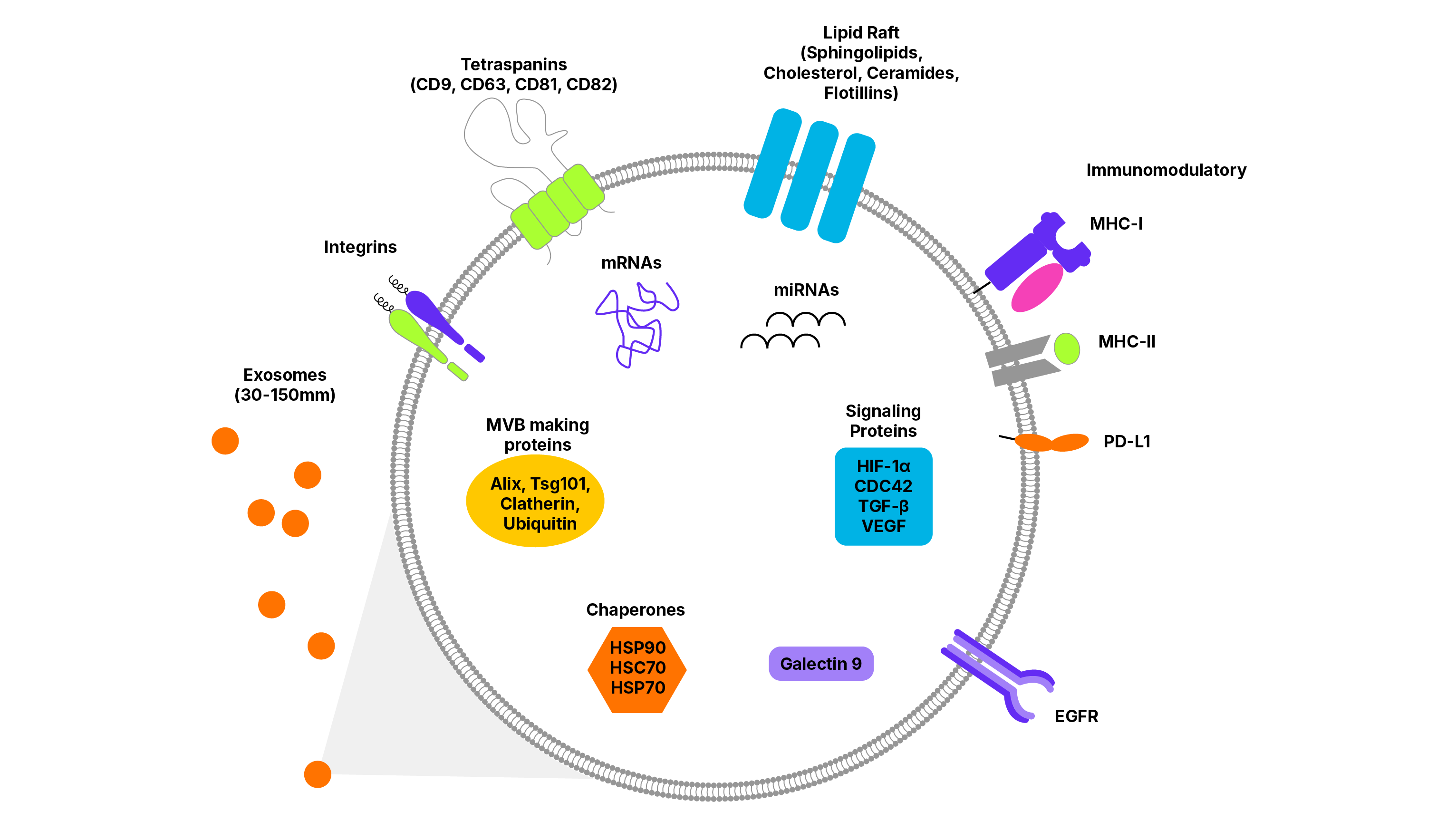
Bio-Techne's extensive offering of exosome marker antibodies, including tissue and tumor-specific exosome marker antibodies are validated for a range of applications, including Simple Western and Flow Cytometry.
| Marker Type | Markers | Function |
|---|---|---|
| Tetraspanins: the most common transmembrane markers to identify exosomes. Proteins in this family have four membrane spanning regions and are localized to tetraspanin enriched microdomains (TEM) in the plasma membrane. | CD9, CD63, CD81, CD82 | Associate with adhesion molecules, transmembrane receptors and intracellular signaling proteins to facilitate and regulate signaling events |
| Multivesicular Body (MVB)-Associated Proteins: can be useful markers for exosome characterization, as they suggest, but do not prove, the intracellular pathway of vesicle biogenesis | ALIX, TSG101 | Interact with proteins of the midbody involved in cytokinesis and membrane scission |
| Flotillin-1 | Scaffolding protein within caveolar membrane, participate in formation of caveolae or caveolae-like vesicles | |
| Clathrin | Involved in receptor-mediated endocytosis and formation of early endosomes | |
| Chaperone Proteins: can be useful exosome marker proteins for a range of environmental and pathophysiological stress conditions | HSP60, HSC70, HSP70, HSP90 | Ensure proper protein-folding and prevent aberrant protein aggregates from building up within a cell |
Protein markers are also important in identifying the cellular origin of EVs; just as individual cell types express identifying markers, exosomes derived from those cells often express cell-type characteristic proteins.
| Cell Type | Exosome Marker |
|---|---|
| Epithelial Cells | EpCAM, CDq47/EMMPRIN, EGFR |
| Lymphocytes (B cell, T cell, NK cells) | CD3, CD19, CD20, CD37, NKG2D, CD56 |
| Mesenchymal Stem/Stromal Cells (MSCs) | MMP-9, CD44, CD73, HGF |
| Platelets | CD31/PECAM-1, CD41, CD62P, PF4, GP-IX (CD42) |
| Neurons | L1CAM, GlueR2, NF-L, TDP43 |
EVs carry out a wide range of functions within the body and their cargo is equally varied. Specific content depends on host species, cellular origin, biofluid, and disease state. Some common markers of signaling, metabolism, cell death, and immunomodulation are listed here
| Function | Markers |
|---|---|
| Signaling | HIF-1α, β-catenin, VEGF, CDC42, ARF1 |
| Metabolic Enzymes | GAPDH, PGK1 |
| Cell Death/ Cytotoxicity | FasL, Granzyme A, Granzyme B |
| Immunomodulatory | HLA-A/B/C, HLA-DR/DP/DQ, TNF-α, CD86, Galectin-9, TGF-β, PD-L1 |
Exosomes and other EVs in Health and Disease
Owing to their critical importance in intercellular communication, exosomes and other EVs, are emerging as important players in disease pathogenesis. EVs have been implicated in diseases such as cancer, Alzheimer’s Disease (AD), and Parkinson’s Disease (PD), as well as a host of other inflammatory pathologies. EVs can transfer cargo to cells in their immediate environment, as well as traverse the networks of blood, lymph, and cerebrospinal fluid (CSF) circulation to deliver messages to distal tissues.
In addition to their role in pathogenesis, exosomes and other EVs are of intense interest as a source for disease biomarkers. Acquiring samples from blood, urine, and CSF to diagnose and monitor disease progression is preferable to traditional invasive biopsy procedures for cancer. For diseases such as Alzheimer’s and Parkinson’s, clinical diagnosis relies heavily on behavioral symptoms, which means there has already been a substantial loss of neurons. Biomarkers to detect disease prior to symptom onset can allow for early interventions to protect neurons and delay symptoms.
Related Research Areas
Resources
- Tools for Isolation, Quantification and Analysis of Exosomes
- Exosome Characterization with Simple Western Protocol
- Liquid Biopsy-Based Biomarkers Whitepaper
- Download our Brochure - Exosomes: Great Things Come in Small Packages
- Next-generation Biomarker Discovery and Diagnostics for Oncology: Exploiting the Potential of Exosomes
- Exosomes: Contents by Cell Type and Process Poster
Background Information
EVs are generally categorized into three main groups based on size, biogenesis, and function.
| Exosomes (small EVs) | Microvesicles (medium/large EVs) | Apoptotic Bodies (large EVs) | |
|---|---|---|---|
| Size | 30-150 nm | 100-1000 nm | Up to 5000nm |
| Biogenesis | Endosomal | Budding | Apoptosis |
| Function | Intercellular communication (varied) | Intercellular communication (varied) | Contain intracellular contents from apoptotic cells |
In place of specific nomenclature relating to biogenesis (e.g. exosome, microvesicle, apoptotic body), the International Society of Extracellular Vesicles (ISEV) recommends referring to EVs based on the following:
| Physical Characteristics | Biochemical Composition | Origin | |
|---|---|---|---|
| Description |
Size (small, medium/large); Density (low, medium, high) |
Specific protein, lipid, or genomic content | Cell type, treatment conditions |
| Examples | Small EVs (sEVs): <200 nm; Medium/large EVs (m/lEVs): >200 nm | CD81+, CD9+, Annexin V+, EpCAM+, EGFR+, miRNAs | Neuronal, epithelial, tumor-derived, hypoxic |
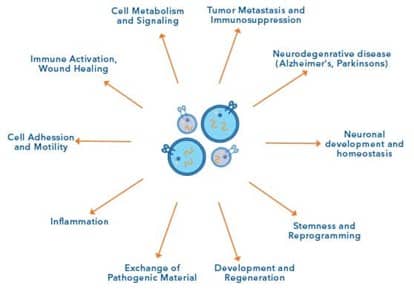
Exosomes and EVs are actively released from many cell types as part of a range of dynamic intercellular signaling processes. Secreted EVs and exosomes may contain a diverse array of cargo necessary for intercellular communication, both locally and in distant tissues. This cargo includes genetic information, such as messenger RNA (mRNA), microRNA (miRNA), non-coding RNA (ncRNA), as well as lipids and many classes of proteins.
EVs including exosomes are key mediators of both intra- and intercellular function, therefore it is unsurprising that they have diverse roles in the human body. For instance, EVs have characterized roles in cellular function, including cell metabolism and signaling, cell adhesion and motility, cell stemness and reprogramming, and neuronal development and homeostasis. In addition, exosomes are implicated in both immunity and disease progression, shown to be involved in tumor metastasis and immunosuppression, inflammation, immune activation and wound healing and the exchange of pathogenic material.
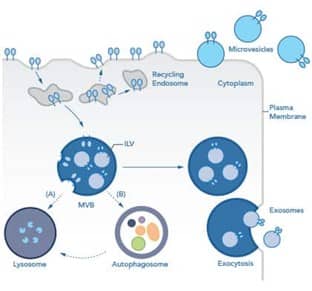
Exosomes are formed in the endosomal maturation pathway. Briefly, inward budding of endosomes into multivesicular bodies (MVBs) results in the formation of intraluminal vesicles (ILVs) within each MVB. There are two possible fates for MVBs: degradation or release. MVBs destined for degradation will either (A) fuse directly with the lysosome or (B) fuse with an autophagosome during the process of autophagy to form an amphisome, which then fuses with the lysosome. MVBs destined for release traffic to the plasma membrane. Upon fusion of MVBs with the plasma membrane, ILVs are released as exosomes via exocytosis. The rate at which exosomes are secreted varies depending on the cellular source, but certain in pathologies, like cancer or hypoxia, an increase in the rate of release of exosomes and other extracellular vesicles is seen.
Microvesicles (MVs), in contrast, are vesicles that are formed directly from the outward protrusions of the plasma membrane and involve cytoskeletal and membrane rearrangement. Both exosomes and MVs are actively secreted from live cells.
Apoptotic bodies are unique EVs, as they are only formed during programmed cell death, or apoptosis, and are rapidly phagocytosed by patrolling macrophages.
There are number of potential applications for exosomes in addition to their role as biomarkers for cancers and other diseases. Their vesicular nature and wide range of functions also make them attractive candidates for therapeutic delivery, regenerative medicine, and vaccine adjuvants.
| Biomarkers |
Proteins and genetic markers found in exosomes can serve as indicators of prognosis and progression for diseases including:
|
|---|---|
| Therapeutic Delivery |
Vesicles carrying small molecules can avoid some of the pitfalls associated with delivery of therapeutics and offer several advantages such as:
|
| Regenerative Medicine |
Mesenchymal stem/stromal cell (MSC)-derived exosomes have the potential to promote tissue repair and regeneration through angiogenesis and cell propagation in preclinical models of:
|
| Vaccine Adjuvants |
Dendritic cell (DC) -derived exosomes are suitable vaccine adjuvants as they can carry peptide-loaded MHC II molecules and activation markers for antigen-specific immune responses. The advantages of these are:
|
- Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018 7 1535750.
- Termini CM, Gillette JM. Tetraspanins function as regulators of cellular signaling. Front Cell Dev Biol 2017:34. https://doi.org/10.3389/fcell.2017.00034.
- Bellin G, Gardin C, Ferroni L, Chachques J, Rogante M, Mitrečić D, et al. Exosome in Cardiovascular Diseases: A Complex World Full of Hope. Cells 2019;8:166. https://doi.org/10.3390/cells8020166.
- Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B. Exosomes, an unmasked culprit in neurodegenerative diseases. Front Neurosci 2017:26. https://doi.org/10.3389/fnins.2017.00026.
- Tao SC, Guo SC, Zhang CQ. Platelet-derived extracellular vesicles: An emerging therapeutic approach. Int J Biol Sci 2017:828–34. https://doi.org/10.7150/ijbs.19776.
- Frühbeis C, Fröhlich D, Krämer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol 2012;3 APR:119. https://doi.org/10.3389/fphys.2012.00119.
- Murphy ME. The HSP70 family and cancer. Carcinogenesis 2013;34:1181–8. https://doi.org/10.1093/carcin/bgt111