By Christina Towers, PhD
Autophagy facilitates the degradation and recycling of damaged cytoplasmic material. The multistep process includes a double membrane structure called an autophagosome that engulfs proteins and organelles and then fuses with lysosomes to facilitate their breakdown. Autophagy is tightly regulated by nutrient availability and is negatively regulated by the robust nutrient sensor mammalian target of rapamycin (mTOR). Starvation, either by glucose deprivation or amino acid depletion, causes a marked increase in autophagy, best quantified by an increase in autophagic flux. Likewise, specific mTOR inhibitors like rapamycin also cause a measurable increase in autophagy.
mTOR-independent mechanisms can also affect autophagy; for example, the naturally occurring disaccharide, trehalose, affects glucose transporters to induce autophagy. Pharmacological interventions have also been identified that can induce autophagy by targeting the upstream autophagophore initiation complex that includes the BH3-containg protein Beclin1. Venetoclax and Tat-Beclin1 are two such interventions.
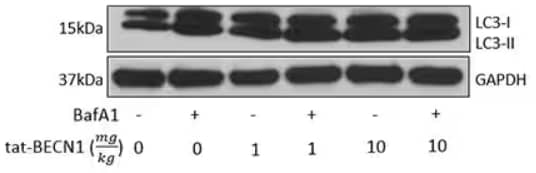
In vivo assay: Tat-Beclin 1 D11 Autophagy Inducing Peptide - Retroinverso form [NBP2-49888] - In vivo dose study in 10wk old C57BL/6J mice. Either 1mg/kg or 10mg/kg IP was administered once daily for 2 days; mice were sacrificed; kidneys prepared for Western blot analysis. Lysosomal inhibitor bafilomycin A1 was used to provide a measurement of autophagic flux. *vehicle is scrambled tat-beclin [NBP2-49887-5mg]. Image from verified customer review.
Autophagy Inhibitors in Cancer Research
Due to the large amount of data supporting a tumor-promoting role of autophagy, there has been a concerted effort to identify and develop autophagy inhibitors. So far, the only agents that have been successfully used in the clinic are antimalarial chloroquine derivatives that target the lysosome, most notably hydroxychloroquine. Recent studies have identified more potent lysomotropic compounds, such as dimeric chloroquine and quinacrines, including DQ661 and Lys05. The vacuolar ATPase inhibitor Bafilomycin A1 also inhibits the lysosome and, therefore, autophagic degradation.
More specific pharmacological inhibitors are being developed that target the upstream regulatory complexes such as ULK1/2 and VPS34 kinase inhibitors as well as the conjugation machinery necessary for efficient autophagosome formation like ATG4b inhibitors (Inducers and inhibitors). These pharmacological and naturally occurring compounds can selectively and robustly affect autophagy; although, there is still a lot of room for improvement. The table below highlights some of the most commonly used compounds for autophagy control, but for a complete list, please refer to the Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition).
Inducers and Inhibitors of Autophagy Process and Their Optimal Doses
| Inducers | Reagents | Best Use to affect Autophagy |
|---|---|---|
| Starvation (Earl Balanced Salt Solution – EBSS) | 1-4hours in culture | |
| Trehalose | 100mM in culture | |
| Tat-Beclin 1 | 5-30µM in culture 15mg/kg i.p. in mice |
|
| Rapamycin | 10-200nM in culture | |
| Venetoclax | 0.2-1µM in culture | |
| Chloroquine | 5-50µM in culture | |
| Hydroxychloroquine | 10-50µM in culture 60mg/kg i.p. daily in mice |
|
| Inhibitors | Bafilomycin A1 | 10nM in culture |
| DQ661 | 3µM in culture 4-8mg/kg i.p. in mice |
|
| Lys05 | 10-100µM in culture 10-80kg/mg i.p. daily in mice |
|
| 3-MA | 1-10mM in culture |

Christina Towers, PhD
University of Colorado (AMC)
Dr. Towers studies the roles of autophagy, apoptosis and cell death in cancer.
-
McAfee, Q. et al. (2012) Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci U S A 109:8253-8258.
-
Rebecca, V. W. et al. (2017) A unified approach to targeting the lysosome's degradative and growth signaling roles. Cancer Discov
-
Towers, C. G. & Thorburn, A. (2016) Therapeutic Targeting of Autophagy. EBioMedicine
-
Tsuboyama, K. et al. (2016) The ATG conjugation systems are important for degradation of the inner autophagosomal membrane Science 354:1036-1041.
-
Sarkar, S. et al. (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641-5652.
-
Liu, Y. et al. (2013) Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc Natl Acad Sci U S A 110:20364-20371.