Carm1 Products
Carm1 (Coactivator-associated arginine methyltransferase 1; also PRMT4) is a 60-64 kDa member of the Arg N-methyltransferase family of enzymes. It is ubiquitously expressed, and found in the cytoplasm during mitosis, and in the nucleus during the G1, G2 and S phases of the cell cycle. Carm1 binds to nuclear receptor p160 family coactivators. When bound, it methylates DNA-associated histone H3 arginines, allowing for chromatin remodeling and gene activation. It also plays a role in pre-mRNA splicing through its methylation of splicing factors, and regulates the stability of RNA-binding proteins. Human Carm1 is 608 amino acids (aa) in length. It contains one catalytic site between aa 184-394, and a transactivation domain at the C-terminus (aa 499-608). There is one automethylation site at Arg550, and a phosphorylation site at Ser216 that, when utilized, promotes cytosolic localization. Carm1 likely forms homodimers. There are three potential isoform variants. One shows an alternative start site at Met378, a second possesses a 16 aa substitution for aa 369-608, and a third contains a deletion of aa 539-561. Over aa 209-379, human and mouse Carm1 are identical in aa sequence.
75 results for "Carm1" in Products
75 results for "Carm1" in Products
Carm1 Products
Carm1 (Coactivator-associated arginine methyltransferase 1; also PRMT4) is a 60-64 kDa member of the Arg N-methyltransferase family of enzymes. It is ubiquitously expressed, and found in the cytoplasm during mitosis, and in the nucleus during the G1, G2 and S phases of the cell cycle. Carm1 binds to nuclear receptor p160 family coactivators. When bound, it methylates DNA-associated histone H3 arginines, allowing for chromatin remodeling and gene activation. It also plays a role in pre-mRNA splicing through its methylation of splicing factors, and regulates the stability of RNA-binding proteins. Human Carm1 is 608 amino acids (aa) in length. It contains one catalytic site between aa 184-394, and a transactivation domain at the C-terminus (aa 499-608). There is one automethylation site at Arg550, and a phosphorylation site at Ser216 that, when utilized, promotes cytosolic localization. Carm1 likely forms homodimers. There are three potential isoform variants. One shows an alternative start site at Met378, a second possesses a 16 aa substitution for aa 369-608, and a third contains a deletion of aa 539-561. Over aa 209-379, human and mouse Carm1 are identical in aa sequence.
| Reactivity: | Human |
| Details: | Mouse IgG Monoclonal Clone #CARM1/7426 |
| Applications: | IHC |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, ICC/IF, KO |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP, KD |
| Reactivity: | Human, Mouse |
| Details: | Goat IgG Polyclonal |
| Applications: | WB, ICC, KO |
| Reactivity: | Human, Rat, Monkey, Primate |
| Details: | Mouse IgG1 Monoclonal Clone #3H2 |
| Applications: | IHC, WB, ELISA, ICC/IF, Flow |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB |
| Applications: | WB, ELISA, MA, AP |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | ICC/IF |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | ICC/IF |
| Applications: | WB |
| Applications: | AC |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, ICC/IF |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, ICC/IF |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, ICC/IF |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, ICC/IF |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP, KD |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP, KD |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP, KD |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, IP |
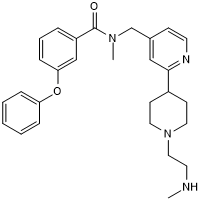
Potent and selective PRMT 4 inhibitor
| Chemical Name: | N-Methyl-N-((2-(1-(2-(methylamino)ethyl)piperidin-4-yl)pyridin-4-yl)methyl)-3-phenoxybenzamide |
| Purity: | ≥98% (HPLC) |
| Reactivity: | Human |
| Details: | Mouse IgG Monoclonal Clone #CARM1/7426 |
| Applications: | IHC |


![Western Blot: Carm1 Antibody [NB100-56374] Knockout Validated: Carm1 Antibody [NB100-56374]](https://resources.bio-techne.com/images/products/Carm1-Antibody-Knockout-Validated-NB100-56374-img0006.jpg)
![Western Blot: Carm1 Antibody [NB200-342] Knockdown Validated: Carm1 Antibody [NB200-342]](https://resources.bio-techne.com/images/products/Carm1-Antibody-Knockdown-Validated-NB200-342-img0013.jpg)


![Western Blot: Carm1 Antibody (3H2)BSA Free [NBP2-37645] Western Blot: Carm1 Antibody (3H2)BSA Free [NBP2-37645]](https://resources.bio-techne.com/images/products/Carm1-Antibody-3H2-Western-Blot-NBP2-37645-img0001.jpg)
![Western Blot: Carm1 Antibody [NB200-341] Western Blot: Carm1 Antibody [NB200-341]](https://resources.bio-techne.com/images/products/Carm1-Antibody-Western-Blot-NB200-341-img0004.jpg)
![Immunohistochemistry-Paraffin: Carm1 Antibody [NBP3-21326] - Immunohistochemistry-Paraffin: Carm1 Antibody [NBP3-21326] -](https://resources.bio-techne.com/images/products/nbp3-21326_rabbit-carm1-pab-45202315531344.jpg)
![Immunocytochemistry/ Immunofluorescence: Carm1 Antibody [NBP2-55993] Immunocytochemistry/ Immunofluorescence: Carm1 Antibody [NBP2-55993]](https://resources.bio-techne.com/images/products/Carm1-Antibody-Immunocytochemistry-Immunofluorescence-NBP2-55993-img0001.jpg)
![Immunocytochemistry/ Immunofluorescence: Carm1 Antibody [NBP2-54924] Immunocytochemistry/ Immunofluorescence: Carm1 Antibody [NBP2-54924]](https://resources.bio-techne.com/images/products/Carm1-Antibody-Immunocytochemistry-Immunofluorescence-NBP2-54924-img0001.jpg)
![Western Blot: Carm1 Overexpression Lysate [NBL1-08698] Western Blot: Carm1 Overexpression Lysate [NBL1-08698]](https://resources.bio-techne.com/images/products/CARM1-Overexpression-Lysate-Adult-Normal-Western-Blot-NBL1-08698-img0002.jpg)