Glyoxalase I Products
Glyoxalase I (also lactoylglutathione lyase, methylglyoxalase, and glx I) is a 21 kDa member of the Glyoxalase I family. The enzyme is an isomerase that catalyzes the formation of S-D-lactoylglutathione from the hemimercaptal adduct that forms spontaneously between methylglyoxal and reduced GSH. The monomeric subunit for human Glyoxalase I is 184 amino acids (aa) in length. In the mature protein, the methionine at the N-terminus is removed. Human Glyoxalase I exists in three separable isoforms as homo-and hetero-dimers of two allelic subunit variants, which differ in charge. The isoforms are formed when residue 19 is changed from cysteine to tyrosine and residue 111 is changed from glutamine to alanine. Each subunit binds one Zn2+ atom. The protein is made up of multiple beta strands and alpha helical regions. Human Glyoxalase I shares 91% and 90% aa sequence identity with rat and mouse Glyoxalase I, respectively. The enzyme is ubiquitously expressed and is also present in many tumor cell lines, in which its concentration is often upregulated. The biological role of the enzyme remains unclear, but the glyoxalase system detoxifies the precursors of advanced glycation end products, which take part in the pathogenesis of vascular, diabetic, and uremic complications.
95 results for "Glyoxalase I" in Products
95 results for "Glyoxalase I" in Products
Glyoxalase I Products
Glyoxalase I (also lactoylglutathione lyase, methylglyoxalase, and glx I) is a 21 kDa member of the Glyoxalase I family. The enzyme is an isomerase that catalyzes the formation of S-D-lactoylglutathione from the hemimercaptal adduct that forms spontaneously between methylglyoxal and reduced GSH. The monomeric subunit for human Glyoxalase I is 184 amino acids (aa) in length. In the mature protein, the methionine at the N-terminus is removed. Human Glyoxalase I exists in three separable isoforms as homo-and hetero-dimers of two allelic subunit variants, which differ in charge. The isoforms are formed when residue 19 is changed from cysteine to tyrosine and residue 111 is changed from glutamine to alanine. Each subunit binds one Zn2+ atom. The protein is made up of multiple beta strands and alpha helical regions. Human Glyoxalase I shares 91% and 90% aa sequence identity with rat and mouse Glyoxalase I, respectively. The enzyme is ubiquitously expressed and is also present in many tumor cell lines, in which its concentration is often upregulated. The biological role of the enzyme remains unclear, but the glyoxalase system detoxifies the precursors of advanced glycation end products, which take part in the pathogenesis of vascular, diabetic, and uremic complications.
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG1 kappa Monoclonal Clone #Glo1a |
| Applications: | IHC, WB, ELISA, ICC/IF, IP, +1 More |
| Source: | E. coli |
| Accession #: | NP_006699 |
| Applications: | EnzAct |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | IHC, WB, ICC/IF |
| Reactivity: | Human |
| Details: | Mouse IgG2B Monoclonal Clone #266 |
| Applications: | IHC, WB, ICC/IF |
Recombinant Monoclonal Antibody
| Reactivity: | Mouse |
| Details: | Rabbit IgG Monoclonal Clone #043 |
| Applications: | ELISA |
Recombinant Monoclonal Antibody
| Reactivity: | Human, Mouse, Rat |
| Details: | Rabbit IgG Monoclonal Clone #3G6Q1 |
| Applications: | WB |
| Reactivity: | Human, Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB, IP |
| Reactivity: | Human |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB, IP |
| Applications: | WB |
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG1 kappa Monoclonal Clone #Glo1a |
| Applications: | IHC, WB, ELISA, ICC/IF, IP, +1 More |
Recombinant Monoclonal Antibody
| Reactivity: | Mouse |
| Details: | Rabbit IgG Monoclonal Clone #043 |
| Applications: | ELISA |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #CPTC-GLO1-3 |
| Applications: | IHC, WB |
Recombinant Monoclonal Antibody
| Reactivity: | Mouse |
| Details: | Rabbit IgG Monoclonal Clone #043 |
| Applications: | ELISA |
| Reactivity: | Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB, ELISA, IP |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #CPTC-GLO1-3 |
| Applications: | IHC, WB |
| Reactivity: | Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB, ELISA, IP |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #CPTC-GLO1-1 |
| Applications: | IHC, WB, MA |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #CPTC-GLO1-1 |
| Applications: | IHC, WB, MA |
| Reactivity: | Mouse |
| Details: | Rabbit IgG Polyclonal |
| Applications: | WB, ELISA, IP |
| Reactivity: | Human |
| Details: | Mouse IgG1 Monoclonal Clone #CPTC-GLO1-3 |
| Applications: | IHC, WB |
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG1 kappa Monoclonal Clone #Glo1a |
| Applications: | IHC, WB, ELISA, ICC/IF, IP, +1 More |
| Reactivity: | Non-species specific |
| Details: | Mouse IgG1 Monoclonal Clone #9F11 |
| Applications: | WB, ELISA, ICC/IF, Flow |
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG1 kappa Monoclonal Clone #Glo1a |
| Applications: | IHC, WB, ELISA, ICC/IF, IP, +1 More |
| Reactivity: | Human, Mouse |
| Details: | Mouse IgG1 kappa Monoclonal Clone #Glo1a |
| Applications: | IHC, WB, ELISA, ICC/IF, IP, +1 More |
| Reactivity: | Human, Mouse, Rat |
| Details: | Goat IgG Polyclonal |
| Applications: | WB, Simple Western |

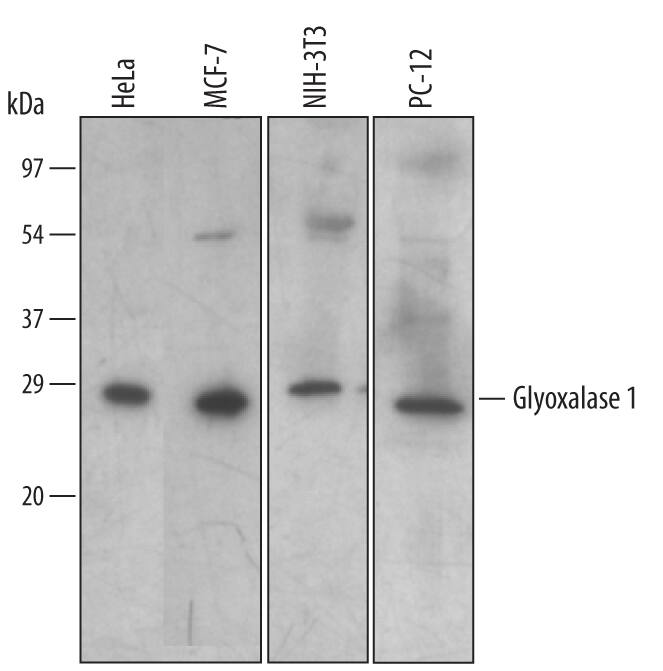
![Western Blot: Glyoxalase I Antibody (Glo1a) [NBP1-19015] Western Blot: Glyoxalase I Antibody (Glo1a) [NBP1-19015]](https://resources.bio-techne.com/images/products/Glyoxalase-I-Antibody-Glo1a-Western-Blot-NBP1-19015-img0007.jpg)


![Western Blot: Glyoxalase I Antibody [NBP1-31466] Western Blot: Glyoxalase I Antibody [NBP1-31466]](https://resources.bio-techne.com/images/products/Glyoxalase-I-Antibody-Western-Blot-NBP1-31466-img0022.jpg)
![Western Blot: Glyoxalase I Antibody (266) [NBP2-43618] Western Blot: Glyoxalase I Antibody (266) [NBP2-43618]](https://resources.bio-techne.com/images/products/Glyoxalase-I-Antibody-266-Western-Blot-NBP2-43618-img0003.jpg)
![Western Blot: Glyoxalase I Antibody (3G6Q1) [NBP3-16375] Western Blot: Glyoxalase I Antibody (3G6Q1) [NBP3-16375]](https://resources.bio-techne.com/images/products/Glyoxalase-I-Antibody-3G6Q1-Western-Blot-NBP3-16375-img0001.jpg)
![Immunoprecipitation:Glyoxalase I AntibodyNBP3-29592] - Glyoxalase I Antibody](https://resources.bio-techne.com/images/products/nbp3-29592_rabbit-glyoxalase-i-pab-3012202412262520.jpg)
![Immunoprecipitation:Glyoxalase I AntibodyNBP3-30500] - Glyoxalase I Antibody](https://resources.bio-techne.com/images/products/nbp3-30500_rabbit-glyoxalase-i-pab-301220241836178.jpg)
![Western Blot: Glyoxalase I Overexpression Lysate [NBL1-11117] Western Blot: Glyoxalase I Overexpression Lysate [NBL1-11117]](https://resources.bio-techne.com/images/products/GLO1-Overexpression-Lysate-Adult-Normal-Western-Blot-NBL1-11117-img0002.jpg)