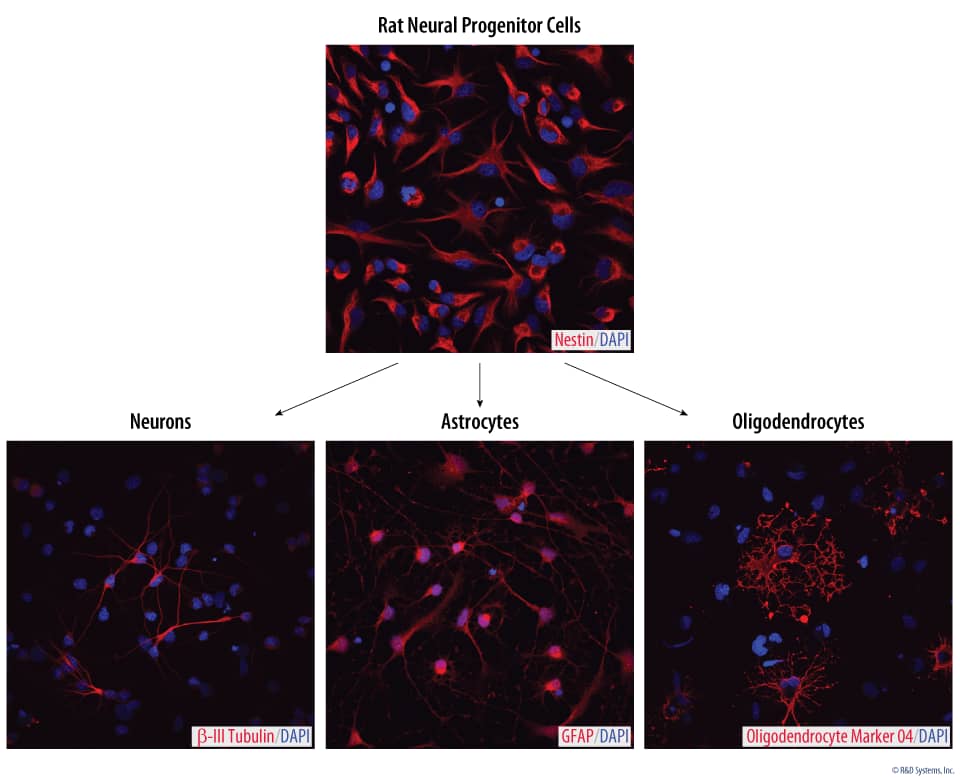
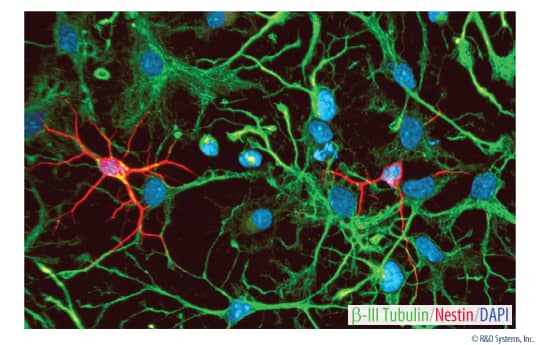
Rat Cortical Stem Cells (3 x 10e6 cells/vial)
R&D Systems, part of Bio-Techne | Catalog # NSC001

Key Product Details
Assay Procedure
Refer to the product datasheet for complete product details.
Reagents & Materials
Reagents Supplied in Rat Cortical Stem Cells (Catalog # NSC001)
- 1 vial of Rat Cortical Stem Cells containing 3 x 106 cells.
Other Supplies Required
Expanding Rat Cortical Stem Cells Using the Neurosphere System
Reagents
- N-2 Plus Media Supplement (Catalog # AR003)
- Recombinant FGF basic (Catalog # 233-FB)
- Bovine Fibronectin (Catalog # 1030-FN)
- PBS
- DMEM/F12
- Glucose
- Glutamine
- NaHCO3
- Penicillin-Streptomycin 100X
- Poly-L-ornithine
- Ca2+/Mg2+-free Hank's Balanced Salt Solution (HBSS) (10X)
- HEPES
- BSA, very low endotoxin
- Trypan Blue, 0.4%
- Deionized (DI) water
Materials
- 10 cm tissue culture plates
- 50 mL centrifuge tubes
- 0.2 µm, sterile filter units
- Plastic cell scraper
- Pipettes and pipette tips
Equipment
- 37 °C and 5% incubator
- Centrifuge
- Hemocytometer
- Microscope
Other Supplies Required
Expanding Rat Cortical Stem Cells Using the Monolayer System
Reagents
- N-2 Plus Media Supplement (Catalog # AR003) or N-2 MAX Media Supplement (Catalog # AR009)
- Recombinant FGF basic (Catalog # 233-FB)
- Bovine Fibronectin (Catalog # 1030-FN)
- PBS
- DMEM/F12
- Glucose
- Glutamine
- NaHCO3
- BSA, very low endotoxin
- Trypan Blue, 0.4%
- Acetic Acid
- Deionized (DI) water
Materials
- 6-well plates
- 15 mL centrifuge tubes
- Pasteur pipettes
- Pipettes and pipette tips
- 0.2 μm, sterile filter unit
Equipment
- 37 °C and 5% incubator
- Centrifuge
- Hemocytometer
- Microscope
Procedure Overview for Expanding Rat Cortical Stem Cells Using the
Neurosphere System
Thawing Cryopreserved Rat Cortical Stem Cells

- Pipette up and down and as cells thaw, transfer the thawed portion into a 50 mL tube containing pre-warmed Completed Base NSC Media supplemented with 20 ng/mL FGF basic.
- Centrifuge the cells at 200 x g for 5 minutes.

- Resuspend the cell pellet in 10 mL of Completed NSC Base Media containing FGF basic.
Neurosphere Expansion

- Perform a cell count.

- Plate cells at approximately 1.0 x 106 NSCs in 5 mL of Completed NSC Base Media supplemented with FGF basic per well in a 6-well plate.
- Incubate the cells at 37 °C and 5% CO2.
- Add fresh FGF basic to the media daily.

- Replace the media every 4 days according to the number of neurospheres present:
- a. Less than 50 neurospheres:
- • Transfer the neurospheres into one well of a 6-well plate using 2.5 mL of fresh media and 2.5 mL of spent/conditioned media.
- b. More than 50 neurospheres:
- • Transfer the media containing the neurospheres to a 15 mL tube.
- • Centrifuge for 5 minutes at 100 x g and remove the media.
- • Resuspend the pellet using a small quantity of fresh Completed NSC Base Media containing FGF basic.
- • Add the neurosphere suspension to 5 mL of fresh Completed Base Media containing FGF basic in one well of a 6-well plate.
- a. Less than 50 neurospheres:
- Passage the cells at 5-6 days or when the neurospheres have a dark clump inside or ruffling on the outside.
Passing Neurosphere

- Transfer the media containing the floating neurospheres to a 15 mL tube.
- Centrifuge for 5 minutes at 100 x g.

- Partially dissociate the neurospheres by pipetting up and down 20 times with a P200 pipette.
- At passage 1 and 2 the cells should be split 1:1. After passage 2 the cells can be split 1:2.
- Add 1 mL of fresh pre-warmed media to the vial of frozen rat cortical stem cells.
- See Details
Procedure Overview for Expanding Rat Cortical Stem Cells Using the
Monolayer System
Thawing Cryopreserved Mouse Cortical Stem Cells

- Add 1 mL of fresh pre-warmed media to the vial of frozen rat cortical stem cells.

- Pipette up and down and as cells thaw.
- Transfer the thawed portion into a 50 mL tube containing pre-warmed Completed Base NSC Media supplemented with 20 ng/mL FGF basic.

- Mix 10 μL of the cell suspension with 10 μL of 0.4% Trypan Blue
- Perform a cell count.
Monolayer Expansion

- Plate 1.0 -1.5 x 106 NSCs in 10 mL of Completed NSC Base Media supplemented with FGF basic onto a Poly-L-ornithine/Fibronectin-coated 10 cm plate.
- Incubate the cells at 37 °C and 5% CO2.

- Replace the media once cells become adherent. After 24 hours, add 10 μL of FGF basic stock (1000X) to the culture.
- Replace the media with fresh Completed NSC Base Media every second day.
- Supplement the media daily with FGF basic.
- Passage the cells when they reach 60-70% confluency.
Passaging Cells

- Wash the cells once with pre-warmed HBSS.
- Add 5 mL of HBSS.
- Incubate at room temperature until the cells round up.

- Scrape the cells from the plate.
- Transfer the cells to a 50 mL centrifuge tube.
- Centrifuge for 5 minutes at 200 x g.
- Remove the supernatant.

- Resuspend the cells in 5 mL of Completed NSC Base Media containing FGF basic.

- Mix 10 μL of the cell suspension with 10 μL of 0.4% Trypan Blue
- Perform a cell count.

- Plate 0.8-1.0 x 106 viable cells in 10 mL of Completed NSC Base Media containing FGF basic onto a Poly-L-ornithine/Fibronectin-coated plate.
- Incubate the cells at 37 °C and 5% CO2.

- Replace the media with fresh Completed NSC Base Media every second day.
- Supplement the media with FGF basic daily.
- Passage the cells after 3 days or when the cells reach 70% confluency.
- Coat cell culture plates with Poly-L-ornithine and Fibronectin.
- See Details
Loading...
Loading...