Introduction to AAV & RNAscope™ | Gene Therapy Probe Design Strategy | Products & Services | Resources
Measure Biodistribution of Your AAV Vector, Transgene mRNA with Spatial and Morphological Context
The RNAscope ISH technology is an ideal solution for detecting adeno-associated virus (AAV) vector DNA and therapeutic transgene mRNA expression with morphological context, addressing critical questions on tissue biodistribution, cellular tropism, and vector performance in vivo. Screen for transduction efficiency of capsid or vectors designed to escape the immune response.
Unlock Answers to Critical Questions
- Characterize tissue biodistribution, cellular tropism, and transduction efficiency of your vector at single-cell resolution in conjunction with cell-type specific markers.
- Measure abundance of AAV+ cells in target tissues and track vector persistence over time.
- Quantify RNA expression of any vector cargo including sequence or codon-optimized human transgenes, CRISPR/Cas9, guide RNAs, or other regulatory non-coding RNAs.
- Demonstrate therapeutic efficacy within vector transduced cells and tissues.
- Easily Scale from small animal models to non-human primates with the capability to readily distinguish human transgenes from homologous host transcripts.
Watch Video (right): Learn How RNAscope Enables Detection of the AAV Vector DNA and Transgene mRNA on the Same Slide

Elevate Your Studies with RNAscope and Unlock a Deeper Understanding of Your AAV Therapeutic and Transgene Expression
Morphology-Based Detection
Morphology-Based Detection
Visualize AAV vector biodistribution and transgene levels at the single-cell level in intact fixed tissue
Targeted Probes
Targeted Probes
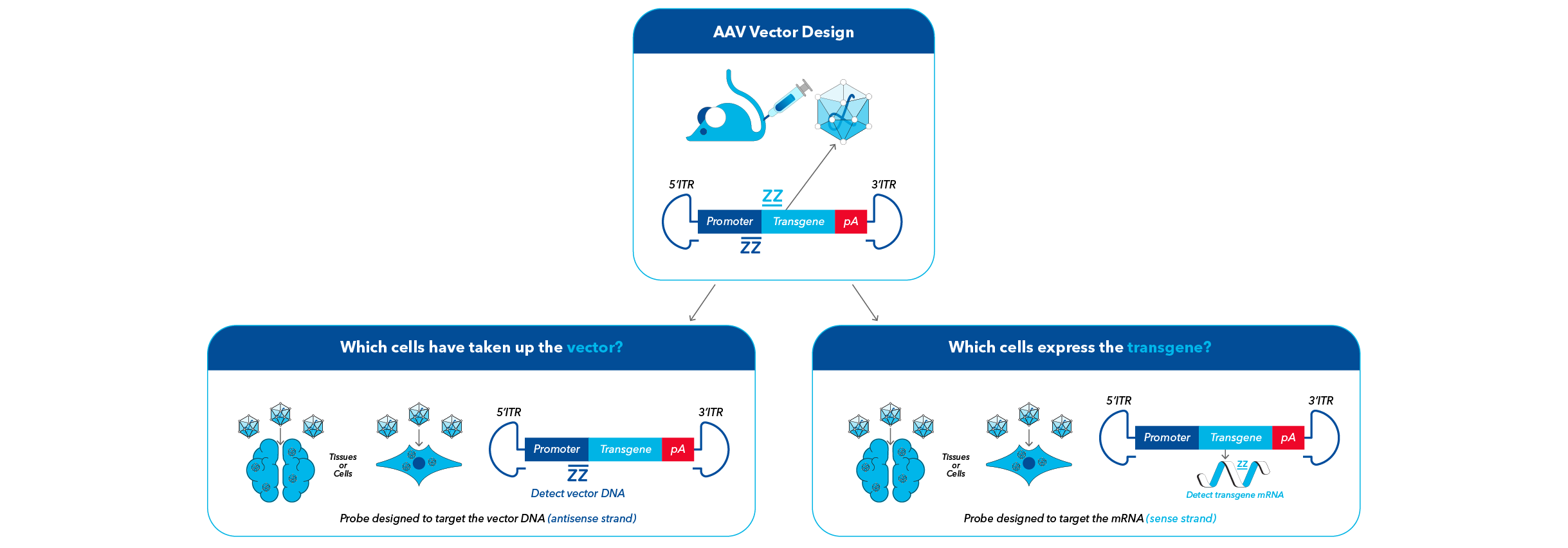
RNAscope probes can be designed to target unique regions within the promoter of the AAV vector DNA or the transgene mRNA of the viral construct
Visualize and Quantify
Visualize and Quantify
Measure percentage of AAV positive cells for vector and transgene expression.
Codon Optimized Expression Analysis
Codon Optimized Expression Analysis
Distinguish transgene from endogenous sequences, at the single nucleotide level.
Gene Therapy Probe Design Strategy
RNAscope probes for AAV-based gene therapy research can be designed to target unique regions within the promotor of the AAV vector DNA or the transgene mRNA of the viral construct.
Customer Success Stories
Customer Webinar
Using BaseScope Assay for Evaluation of Ocular Biodistribution of Gene Therapy Products
Customer Webinar Highlight (ASGCT 2023)
Julio D Nieves, Associate Director, Imaging, Adverum
Visualize the AAV Vector DNA and the Transgene mRNA on the Same Slide
Image of the Naive and Transduced Region of the Mouse Retina
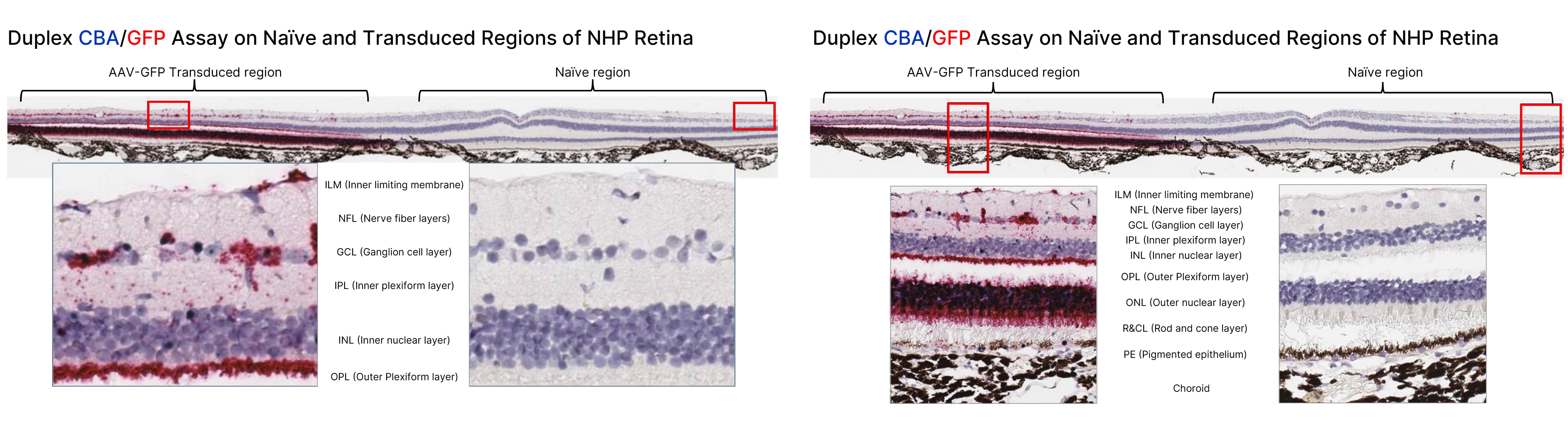
Fig 1. Identification of specific cells transduced by AAV-GFP following subretinal injection using the RNAscope 2.5 HD Duplex assay to simultaneously detect the CB promoter DNA sequence of the AAV vector (green) and the GFP transgene mRNA (red). Staining was observed in almost all of the retinal layers (with the exception of the choroid) in the transduced region, but no staining was observed in the non-transduced naïve region.
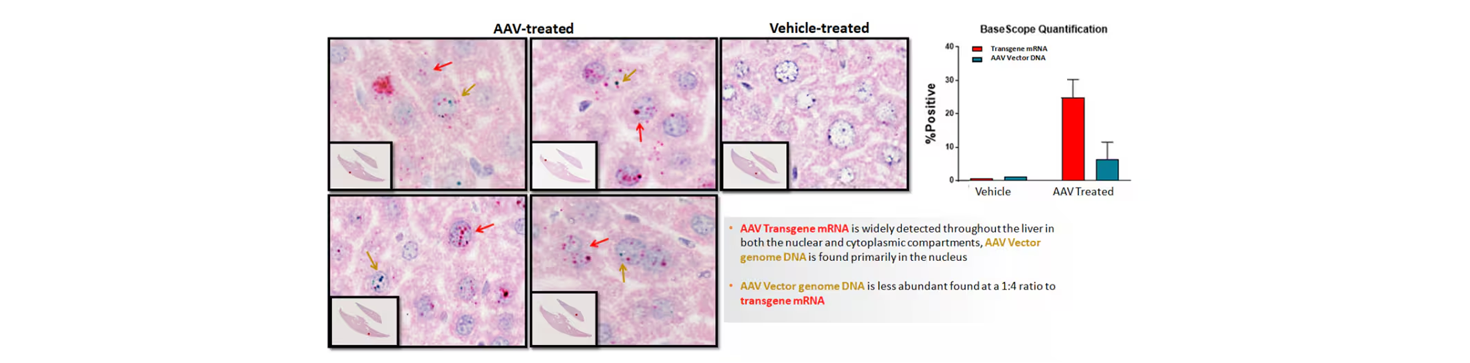
Fig 2. BaseScope staining allows visualization and quantification of transgene expression (red arrow) and AAV vector (green arrow) presence in treated liver samples
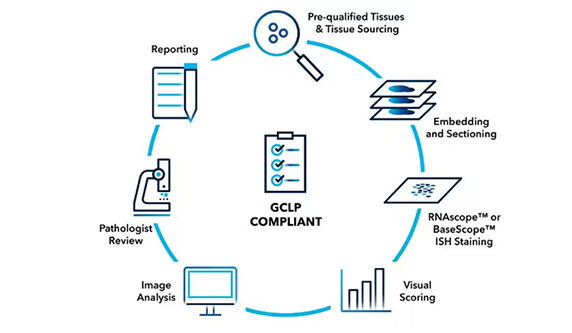
RNAscope™ Professional Assay Services
Our Professional Assay Services offer you direct access to the developers of RNAscope in situ hybridization (ISH) technology.
Accelerate your journey in target validation, preclinical, and clinical development with the promise that we will provide consistent and trustworthy data to drive your projects forward.
RNAscope ISH Technology - Product Formats
Multiomic Spatial Solutions for RNA and Protein Co-detection
RNAscope~20 ZZ probe pairs Designed to > 300 nt.
| BaseScope™1-3 ZZ probe pairs Designed to ~ 50-300 nt.
| miRNAscope™Designed to ~17-50 nt.
| RNAscope PlusRNA species of varied sizes.
|
Featured Resources For AAV-based Gene Therapy
Reference
- S12 Nonclinical Biodistribution Considerations for Gene Therapy Products; Guidance for Industry; U.S. Department of Health and Human Services Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER) May 2023; ICH-Safety.