G protein-coupled receptors (GPCRs) utilize trimeric G proteins to transduce information from the extracellular environment and other cells to intracellular signals that modulate cellular responses.
Table of Contents

GPCR Product Ranges
Search for your GPCR target and product category in the above search bar, or browse below. If you can't find what you are looking for, please Contact Us and we'll be happy to help.
| Antibodies | Agonists, Antagonists & Peptides | cDNA Clones | Proteins |
| Fluorescent Dyes and Probes | Proteome Profiler Antibody Arrays | PROTAC® Degraders | Custom Antibody Services |
GPCR Background and Product Highlights
GPCR Families and Structures | GPCR Signaling | Regulation of GPCR Signaling | Physiological Functions of GPCRs
G protein-coupled receptors (GPCRs) utilize trimeric G proteins to transduce information from the extracellular environment and other cells to intracellular signals that modulate cellular responses. They are not only the largest and most diverse family of cell membrane receptors, but are, in fact, one of the largest protein classes in mammals. With more than 800 genes in this receptor family, and binding to a vast array of ligands, GPCRs play a role in multiple physiological functions including sight, taste, smell, neurotransmission, pain perception, and immune responses. Novus offers a broad selection of GPCR antibodies covering approximately 80% of the 800+ GPCRs identified and are extensively validated using the 5 Pillars of Antibody Validation. Explore our antibodies.
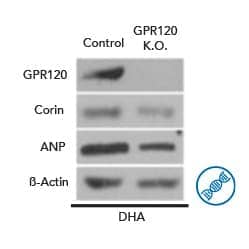
Knockout Validation of FFAR4/GPR120 Antibody. Corin and ANP were measured in 3T3-L1 control and GPR120 knockout (K.O.) cells that were exposed to DHA (100 i1/4M) for 2 days in the presence of differentiation medium. A Rabbit Anti-Human/Mouse/Rat FFAR4/GPR120 Antigen Affinity-Purified Polyclonal Antibody (Catalog # NBP1-00858) was used to verify the presence and absence of FFAR4/GPR120 in the cultured cells. Image from Bae, I.S. and S.H. Kim (2019) Int. J. Mol. Sci. 20:6128. Image licensed under a CC-BY license.
GPCRs are also involved in numerous diseases such as cancer, inflammatory and cardiac disorders, and metabolic diseases. As such, these receptors are highly targeted for drug development. About 30-40% of approved drugs target GPCRs. However, this represents only a small proportion of human GPCRs, so there is enormous potential for new drug development targeting these receptors.
While the amino acid sequence of GPCRs differs greatly, all GPCRs share a common core structure. They are composed of seven transmembrane α-helices (TM1 – TM7) that are connected by three intracellular (ICL) and three extracellular (ECL) loops, an extracellular N-terminus, and an intracellular C-terminus. The helices of the GPCR are arranged into a tertiary structure that resembles a barrel, forming a cavity within the plasma membrane. An additional helix, Helix 8, important for surface expression of GPCRs, receptor trafficking, and G protein activation and specificity, is in the C-terminal domain.

GPCRs are commonly categorized into different subfamilies based on structural and physiological features of the protein. This classification system, the A-F system, divides GPCRs into six groups, four of which contain mammalian GPCRs: Class A Rhodopsin-like, which contains approximately 80% of GPCRs; Class B Secretin-like; Class C Metabotropic Glutamate Receptors; and Class F Frizzled/Smoothened. The Class D and Class E families are composed of non-mammalian GPCRs. The Class D family are fungal mating pheromone receptors while the Class E family contains cAMP receptors from slime molds.
In recent years, another classification system for mammalian GPCRs has been proposed. The GRAFS System divides mammalian GPCRs into 5 families [Glutamate (G), Rhodopsin (R), Adhesion (A), Frizzled/Taste2 (F), Secretin (S)] based on their phylogenetic tree. The main difference between the A-F and GRAFS system is that the latter separates the Class B GPCR family into 2 groups: the Secretin family and the Adhesion family.

Despite these similarities, the separate GPCR classes exhibit structural differences, largely within the extracellular N-terminal domain (ECD) and ligand-binding sites. For instance, the ligand binding site for Class A GPCRs is located within the extracellular region of the barrel that is formed by the transmembrane domains. However, the primary ligand-binding site for Class B GPCR families, which bind to large peptides, is in an extra-large ECD.
Class B2 GPCRs have several protein motifs in their ECD, such as epidermal growth factor, cadherin, and immunoglobulin domains, that facilitate the protein-protein interactions necessary for cell adhesion and migration. This GPCR family also contains a GPCR autoproteolysis-inducing (GAIN) domain and the GPCR proteolytic site (GPS) that are responsible for receptor activation.
Class C GPCRs possess a large, distinctive ECD that is divided into a ligand-binding domain (LBD) and a cysteine-rich domain (CRD, except for the GABAB receptor). The LBD contains a Venus flytrap module (VFTM), which is a bilobed structure that contains the orthosteric binding site within its crevice. Class C GPCRs also differ from other GPCR families in that they must form hetero- or homodimers for activation of signaling pathways. The ECD of Class F GPCRs contains a CRD, which is linked to the TMs with a linker domain (LD).
GPCR Signaling
G proteins consist of two functional units, an α subunit (Gα) and a βγ complex (Gβγ). The Gα subunit, a monomeric GTPase, contains a binding site for guanosine nucleotides. When Gα is bound to GDP, the G proteins are inactive, attached to each other, and connected to GPCRs. Ligand binding to the receptors, which are also guanine nucleotide exchange factors (GEFs), activates the Gα subunit, triggering the exchange of GDP for GTP.
This causes the G protein to detach from the GPCR and the two units to dissociate. Each functional unit independently acts on effector molecules to activate downstream signaling pathways. Signaling will continue until the GTP molecule is hydrolyzed by the Gα subunit. Additionally, the signal is amplified as the GPCR will continue to activate G proteins for as long as the ligand is bound. Once GTP is hydrolyzed to GDP, Gα-GDP binds to the Gβγ dimer and the heterotrimeric G protein reassociates to the GPCR.
GPCR signaling is very complex, allowing for the activation of a huge diversity of signaling pathways that regulate many different cellular functions. This complexity is due to numerous factors including multiple receptor activation states, receptor dimerization, and activation of non-G protein effector molecules. Additionally, hundreds of different subunit combinations for heterotrimeric G proteins exist due to the vast repertoire of Gα, Gβ, and Gγ isoforms. Each G protein subunit combination binds to specific GPCRs and affects specific effector molecules.

Small Molecules and Peptides for GPCRs
We offer a large catalog of compounds targeting GPCRs including agonists, antagonists, allosteric modulators, inhibitors, Degraders, and fluorescent dyes and probes.
Regulation of GPCR Signaling
GPCR signaling is terminated by hydrolysis of the Gα-bound GTP molecule. However, several mechanisms exist to regulate GPCR signaling. Regulators of G Protein Signaling (RGS) is a diverse family of 20+ proteins that negatively regulate GPCR signaling. They directly interact with Gα subunits, particularly Gαi, Gαo, and Gαz, to accelerate GTP hydrolysis and terminate signaling.
GPCR signaling can also be terminated through a two-step mechanism that involves GPCR kinases (GRKs) and arrestin proteins. GRKs are serine/threonine protein kinases that phosphorylate multiple sites on the C-terminus of activated GPCRs. Arrestin proteins bind to the phosphorylated C-terminus and block the binding of additional G proteins. Arrestins also interact with components of coated pits to recruit GPCRs to the pits and promote Dynamin-dependent receptor internalization. Internalized GPCRs are then dephosphorylated and either recycled back to the cell membrane or targeted for degradation.
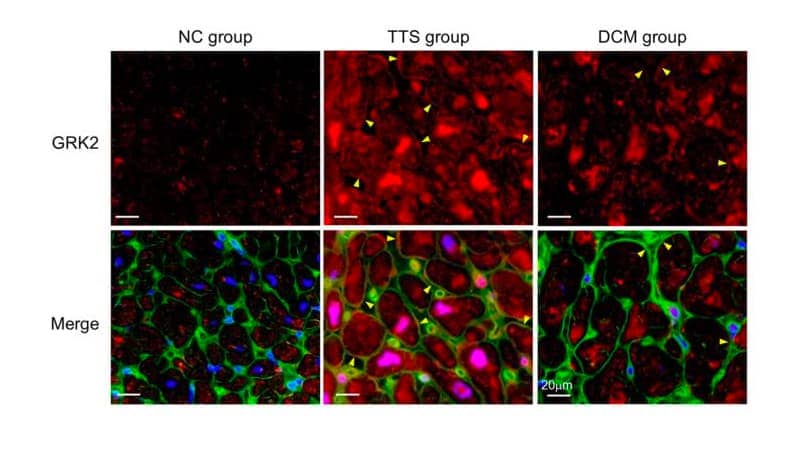
GRK2 Expression in Myocardial Tissue with TTS. GRK2 expression was measured in myocardial tissue from individuals with human takotsubo syndrome (TTS) or dilated cardiomyopathy (DCM), or normal controls (NC) using a Mouse Anti-Human/Mouse/Rat/Primate GRK2 Monoclonal Antibody (Catalog # NBP2-37611; red). GRK2 expression was observed in the cytoplasm and cell membrane. Cell membrane stained with wheat germ agglutinin staining (green). Nucleus stained with DAPI (blue; Catalog # 5748). Image from Nakano, T. et al. (2018) Sci. Rep. 8:12731. Image licensed under a CC-BY license.
Physiological Functions of GPCRs
Since GPCRs are widely distributed and trigger cellular responses to a broad variety of extracellular stimuli, they play key roles in most physiological functions and are associated with numerous pathological conditions. About half of the 800 GPCRs mediate sensory information such as pheromone sensation, olfaction, light perception, and taste. Of the remaining GPCRs, more than 90% are expressed in the brain.
GPCRs in the Nervous System
In the vertebrate nervous system, GPCRs are responsible for slow synaptic transmission, i.e., synaptic transmission that involves that activation of a series of biochemical events. Both the major excitatory and inhibitory neurotransmitters, glutamate and GABA, respectively, and a preponderance of other neurotransmitters, including acetylcholine, adenosine, dopamine, serotonin, histamine, ATP, adrenaline/noradrenaline, endocannabinoids, enkephalins/endorphins, and neuropeptides, signal through GPCRS.
GPCRs located on the presynaptic membrane regulate the release of neurotransmitters while postsynaptic GPCRs can control gene expression and influence the efficiency of ionotropic receptors. Nervous system GPCRs play important roles in mood, cognition, pain, and appetite, and are involved in many neurodegenerative and psychiatric disorders.
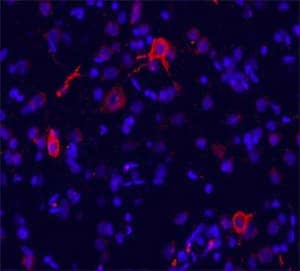
mGluR1 Expression in Rat Brain. Metabotropic Glutamate Receptor 1 (mGluR1) was detected in rat brain (cingulate cortex) using a Rabbit Anti-Human/Mouse/Rat/Chicken mGluR1 Affinity-Purified Polyclonal Antibody (Catalog # NB110-39033) followed by a Cy3-conjugated anti-rabbit secondary antibody (red). The tissue was counterstained with DAPI (blue; Catalog # 5748).
GPCRs in the Immune System
GPCRs are expressed by many inflammatory cells, including dendritic cells, monocytes, lymphocytes, and neutrophils, and play important roles in the immune system. Chemokine receptors are the signature GPCR subfamily associated with immune functions as they mediate the controlled migration of immune cells during innate and adaptive immunity.
However, many other GPCR subfamilies, including adhesion GPCRs, purinergic receptors, adenosine receptors, formylpeptide receptors, and Lysophospholipid S1P and LPA receptors, are important for immune responses as they regulate activities such as immune cell differentiation and maturation, phagocytosis, and secretion of antimicrobial compounds. Aberrant GPCR expression or activation underlies the pathogenesis of many autoimmune diseases, immunodeficiencies, and autoinflammatory syndromes.
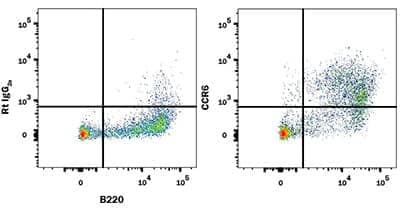
Detection of CCR6 in Mouse Splenocytes. Mouse splenocytes were stained with a Fluorescein-Conjugated Rat Anti-Mouse B220/CD45R Monoclonal Antibody (Catalog # FAB1217F) and a (top graph) Rat Anti-Mouse CCR6 Monoclonal Antibody (Catalog # MAB590) or a (bottom graph) Rat IgG2A Isotype Control Antibody (Catalog # MAB006) followed by an APC-Conjugated Goat anti-Rat IgG Secondary Antibody (Catalog # F0113).
GPCRs in Cancer
GPCRs are involved in many cancer types. They are not only expressed by tumor cells, but also by many cells present in the tumor microenvironment including immune, stromal, and vascular cells. GPCRs and GPCR-mediated signaling regulate many cellular functions vital for cancer progression such as tumor cell proliferation, invasion, migration, and metastasis. Research has identified altered GPCR signaling in many cancers due to aberrant overexpression, gain-of-function mutations, mutations in downstream effector molecules, and increased production of GPCR ligands by tumor and stromal cells.
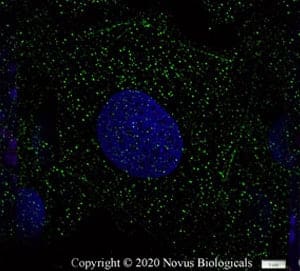
GPR18 in Ntera2 Cells. GPR18 was detected in fixed Ntera2 human embryonal carcinoma cells using a Rabbit Anti-Human/Mouse/Rat GPR18 Antigen Affinity-Purified Polyclonal Antibody (Catalog # NBP2-24918). Cells were stained with a Dylight 488-conjugated anti-rabbit secondary antibody (green) and counterstained with DAPI (blue; Catalog # 5748).
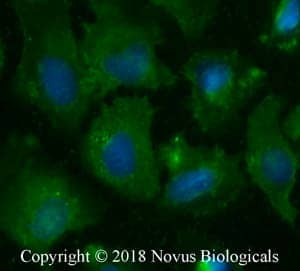
SSTR2 in U2OS Cells. Somatostatin R2 (SSTR2) was detected in formalin-fixed U2OS human osteosarcoma epithelial cells using a Rabbit Anti-Human/Mouse/Rabbit/ Porcine Somatostatin R2/SSTR2 Antigen Affinity-Purified Polyclonal Antibody (Catalog # NB300-157). Cells were stained with a Dylight 488-conjugated anti-rabbit secondary antibody (green) and counterstained with DAPI (blue; Catalog # 5748).
Arakaki, A.K.S., Pan, W.A. and Trejo, J. (2018) GPCRs in Cancer: Protease-Activated Receptors, Endocytic Adaptors and Signaling. Int. J. Mol. Sci. 19:1886. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6073120/
Betke, K.M., Wells, C.A. and Hamm, H.E. (2012) GPCR Mediated Regulation of Synaptic Transmission. Prog. Neurobiol. 96:304. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3319362/
de Oliveira, P.G., Ramos, M.L.S., Amaro, A.J., Dias, R.A. and Vieira, S.I. (2019) Gi/o-Protein Coupled Receptors in the Aging Brain. Front. Aging Neurosci.11:89. https://www.frontiersin.org/articles/10.3389/fnagi.2019.00089/full.
Dubyak, G.R. (2020) Chapter 21 – GPCRs in Innate and Adaptive Immune Response. In: Jastrzebska, B. and Park, P.S.H. (eds) GPCRs. Academic Press. https://doi.org/10.1016/B978-0-12-816228-6.00021-0.
Gurevich, V.V. and Gurevich, E.V. (2019) GPCR Signaling Regulation: The Role of GRKS and Arrestins. Front. Pharmacol. 10:125. https://www.frontiersin.org/articles/10.3389/fphar.2019.00125/full
Higler, D., Masureel, M. and Kobilka, B.K. (2018) Structure and Dynamics of GPCR Signaling Complexes. Nat. Struct. Mol. Biol. 25:4. https://www.nature.com/articles/s41594-017-0011-7
Huang, Y. and Thathiah, A. (2015) Regulation of Neuronal Communication by G Protein-Coupled Receptors. FEBS Letters. 589:1607. https://www.sciencedirect.com/science/article/pii/S0014579315003609.
Insel, P.A., Sriram, K., Gorr, M.W., Wiley, S.Z., Michkov, A., Salmerón, C. and Chinn, A.M. (2019) GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharm. Sci. 40:378.
https://www.sciencedirect.com/science/article/abs/pii/S0165614719300641
Kahsai, A.W., Pani, B. and Lefkowitz, R.J. (2018) GPCR Signaling: Conformational Activation of Arrestins. Cell Res. 28:783. https://www.nature.com/articles/s41422-018-0067-x
Lin, H.H., Hsiao, C.C., Pabst, C., Hébert, J., Schöneberg, T. and Hamann, J. (2017) Chapter 5 – Adhesion GPCRs in Regulating Immune Responses and Inflammation. In: Shukla, A.K. (eds) Advances in Immunology. Academic Press. https://doi.org/10.1016/bs.ai.2017.05.005
Packiriswamy, N. and Parameswaran, N. (2015) G-Protein-Coupled Receptor Kinases in Inflammation and Disease. Genes Immun. 16:367. https://www.nature.com/articles/gene201526
Senese, N.B., Kandasamy, R., Kochan, K.E. and Traynor, J.R. (2020) Regulator of G-Protein Signaling (RGS) Protein Modulation of Opioid Receptor Signaling as a Potential Target for Pain Management. Front. Mol. Neurosci. 13:5. https://www.frontiersin.org/articles/10.3389/fnmol.2020.00005/full
Siderovski, D.P. and Kimple, A.J. (2018) RGS Protein Family. In: Choi S. (eds) Encyclopedia of Signaling Molecules. Springer, Cham. https://doi.org/10.1007/978-3-319-67199-4_527
Usman, S., Khawer, M., Rafique, S., Naz, Z. and Saleem, K. (2020) The Current Status of Anti-GPCR Drugs Against Different Cancers. J. Pharm. Analysis. 10:517. https://www.sciencedirect.com/science/article/pii/S2095177919302874
van Gastel, J., Hendrickx, J.O., Leysen, H., Santos-Otte, P., Luttrell, L.M., Martin, B. and Maudsley, S. (2018) β-Arrestin Based Receptor Signaling Paradigms: Potential Therapeutic Targets for Complex Age-Related Disorders. Front. Pharmacol. 9:1369. https://www.frontiersin.org/articles/10.3389/fphar.2018.01369/full
Zhou, Q., Yang, D., Wu, M., Guo, Y., Guo, W., Zhong, L., Cai, X., Dai, A. and Jang, W. (2019) Common Activation Mechanism of Class A GPCRs. eLife 8:e50279. https://elifesciences.org/articles/50279
PROTAC® is a registered trademark of Arvinas Operations, Inc., and is used under license.