Gut-Brain Axis
What is the Gut-Brain Axis?
The gut-brain axis refers to the bidirectional communication between the nervous system of the gastrointestinal (GI) tract, termed the enteric nervous system (ENS), and the central nervous system (CNS). The adult human gut contains more than 1 kg of bacteria, which are not only responsible for intestinal health but also influence the functioning of other organs, including the brain.
Gut microbiota are capable of communicating with the brain through a variety of routes, including via direct pathways of the spinal cord and vagal nerve or through circulating metabolites and immune cells. The gut microbiome plays a critical role in many neurogenerative processes such as the formation of the blood-brain barrier, myelination, microglia maturation, the immune response to infection, and neurogenesis. Altered or dysregulated gut microbiota, especially in early childhood or during aging, can have a severe effect on brain function, leading to neuropsychiatric conditions and neurodegenerative disorders.
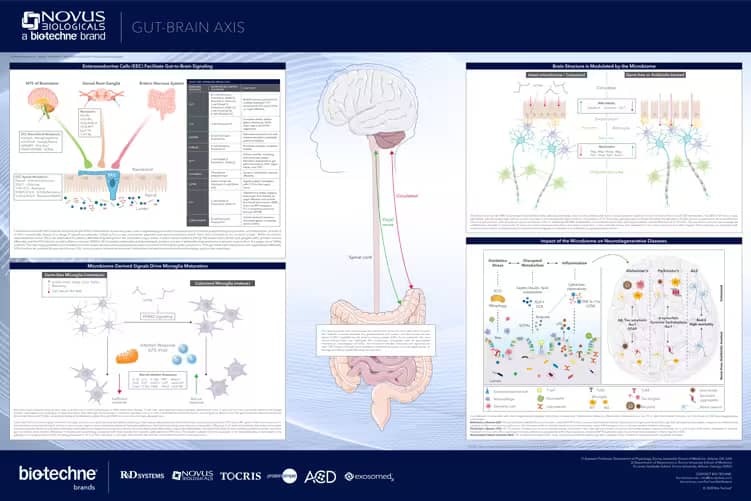
Gut-Brain Axis Poster
Learn more about the gut-brain axis in this poster, developed in collaboration with Dr. Timothy Sampson, PhD from the Department of Physiology at Emory University School of Medicine. Key highlights include:
- An overview of the neuro-epithelial interface in the gut
- The impact of gut-derived signals on neuroinflammation and neurodegenerative diseases
- The alteration of brain structure in response to gut-derived signals
- The effect of the gut microbiome on microglia homeostasis and activation
Enteric Nervous System
The enteric nervous system (ENS) is part of the autonomic nervous system that can function independently but also has multiple connections with the CNS, referred to as the gut-brain axis. The enteric nervous system, as depicted below, is a complex series of sensory neurons, interneurons and motor neurons that form local circuitry within the enteric wall, with inputs from both the local environment and the CNS.
Key Players and Neuronal Interactions of the Enteric Nervous System
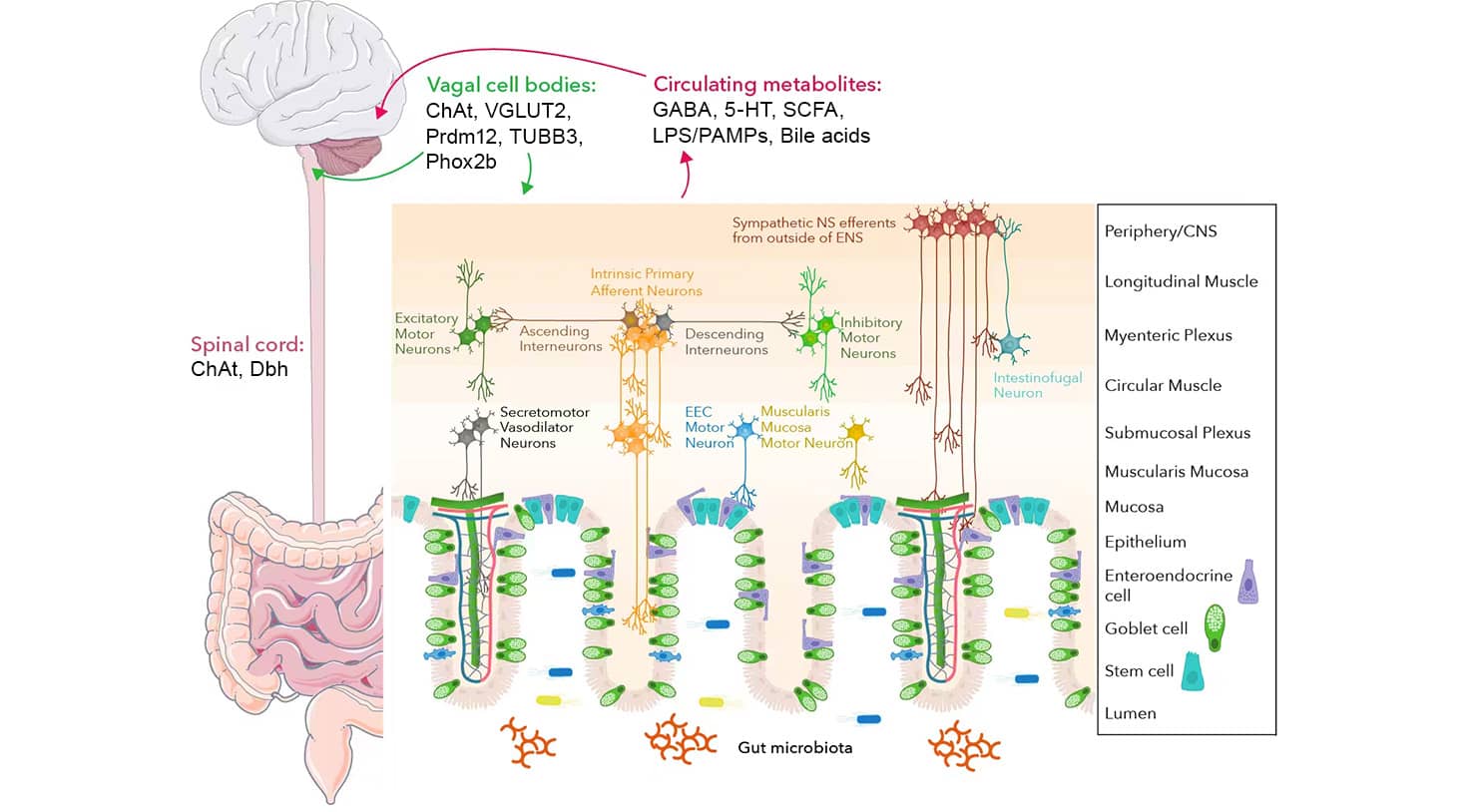
Graphic created in collaboration with Dr. Timothy Sampson, PhD from the Department of Physiology at Emory University School of Medicine
GI motility is regulated by the interactions between enteric neurons, muscles, and intestinal epithelial cells. Among these, the sensory neurons Intrinsic Primary Afferent Neurons (IPANs) play a central role in mechanosensing and respond to chemical stimuli to regulate motility. For instance, microbial-derived and host-produced molecules, including lipopolysaccharide (LPS) and bile acids, are secreted into circulation, detected by sensory neurons, and processed by the brain.
The vagus nerve and spinal cord also express important proteins and enzymes including choline acetyltransferase (ChAT), which regulates the biosynthesis of the neurotransmitter acetylcholine, and βIII tubulin (TUBB3), which plays a role in neuronal development. The vagus nerve contains afferent (sensory) and efferent (motor) fibers and is therefore responsible for both carrying motor signals to the gut and relaying sensory cues back to the CNS.
Another key enteric nervous system player is EECs, often referred to as the taste cells of the gut, which are specialized intestinal epithelial cells capable of sensing and responding to environmental signals. EECs play a role in gut-brain communication through direct and indirect neuronal connections, serving as a vital component of the enteric nervous system.
Three-dimensional (3D) cell cultures, also called organoids, are becoming the preferred technology for studying the complex, dynamic nature of the enteric nervous system compared to traditional models involving animal models, post-mortem tissue analysis, and human genetics studies. 3D cell culture model systems more accurately recapitulate cell-cell interactions compared to 2D cell culture models while also providing access to previously inaccessible tissues. Enteric organoids consist of human intestinal organoids with the addition of enteric and sensory neuron inputs.
Enteroendocrine Cell (EEC) Signaling
EECs play a role in regulating secretion and gut motility, food intake, glucose levels following meals, and metabolism. EECs can sense luminal content and in turn produce and secrete signaling molecules like hormones, peptides, and neurotransmitters that can act on local circuits and bind specific receptors. EECs can transmit signals locally to other nearby intestinal cells through paracrine signaling, through circulating hormones in the bloodstream via endocrine signaling, and through neuronal pathways.
The enteric neuroepithelial circuit is formed by axon-like processes making direct connections from the EECs to the brain and gut. The EECs are innervated by sensory afferents from various locations, forming the bidirectional brain-gut communication. Specifically, there are neuronal processes from the brainstem (vagus nerve, nucleus tractus solitarius (NTS)), the dorsal root ganglia (DRG) of the spinal cord, and the enteric nervous system (ENS).
EECs Facilitate Gut-to-Brain Signaling
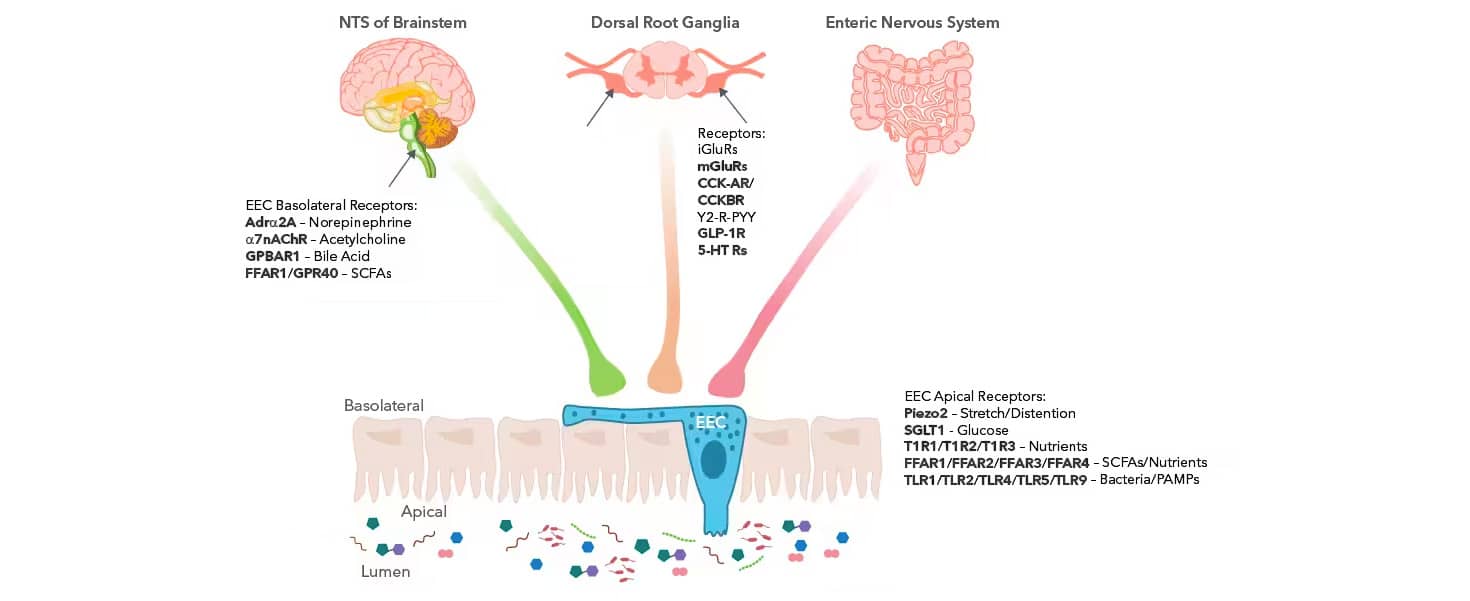
Graphic created in collaboration with Dr. Timothy Sampson, PhD from the Department of Physiology at Emory University School of Medicine.
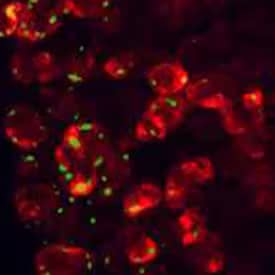
CCK-A R was detected in perfusion fixed frozen sections of rat pancreas using 5 µg/mL Human/Mouse/Rat CCK-A R Antigen Affinity-purified Polyclonal Antibody (AF2680) overnight at 4 °C. Tissue was stained (red) and counterstained (green).
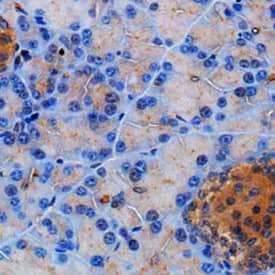
IHC staining with Somatostatin Receptor 2/SSTR2 Antibody [NB300-157] in mouse pancreas using DAB with hematoxylin counterstain.
The various receptors located on the EECs themselves and within the NTS and DRG are essential for gut-CNS communication and are responsible for sensing the secreted metabolites and molecules and relaying appropriate responses. EECs possess hormone-containing basal cytoplasmic processes called neuropods that can extend to distant cells and connect directly to nerves. These neuropods help form synaptic connections between the presynaptic and postsynaptic proteins from the EECs and the sensory neurons.
There are distinct subpopulations of EECs that release specific signaling molecules and hormones in response to diverse stimuli. Transgenic mouse studies have revealed that EECs are capable of secreting more than one signaling molecule and have complex co-expression profiles.
The signaling molecules produced by EECs are also influenced by the region of the GI tract. For instance, A cells, one EECs subtype located primarily in the stomach, release the signaling molecule ghrelin, while L cells largely located in the distal small intestine (SI) and colon produce 5-HT, GLP-1, and Peptide YY (PYY). These signaling molecules have diverse functions ranging from GI motility to signaling satiety. Identifying the specific signaling molecules expressed by each cell subtype will result in better characterization of EECs and a provide a more thorough understanding of cell-molecule-receptor interactions.
EEC Signaling Molecules, Cell Subtype, Receptor Specificity and Location, and Function
| EEC Signaling Molecule | Signaling Molecule Function | EEC Subtype (Location) | Specific Receptor | Receptor Localization |
|---|---|---|---|---|
| 5-HT | Multifunctional, promotes GI motility; activates 5-HT receptors (5-HT3 and 5-HT4) on vagal afferents | EC-Cell (Stomach Proximal SI ,Distal SI, Proximal LI, Distal LI) L-Cell (Distal SI, Proximal LI, Distal LI) I-Cell (Proximal SI) K-Cell (Proximal SI) |
5-HT Receptors | CNS, Enteric neurons, Immune cells, Muscle cells, Vagal nerve |
| Cholecystokinin (CCK) | Increases satiety, delays gastric emptying; responsive to SCFAs | I-Cell (Proximal SI) | CCK-AR CCKBR |
CNS, Enteric neurons, Gallbladder, GI tract, Pancreas, Vagal nerve |
| Gastrin | Stimulates stomach acid and enzyme secretion, promotes gastric emptying | G-Cell (Stomach, Proximal SI) | CCKBR | CNS, GI tract |
| Ghrelin | Promotes appetite, increases motility | G-Cell (Stomach, Proximal SI) | Ghrelin Receptor/ GHSR | CNS, Vagal nerve |
| GLP-1 | Inhibits motility, emptying, and promotes satiety | L-Cell (Distal SI, Proximal LI, Distal LI) | GLP-1R | CNS, Enteric neurons, Immune cells, Kidney, Pancreas |
| Glutamate | Synaptic signaling to sensory afferents | Throughout intestinal tract | iGluR mGluR |
CNS |
| Leptin | Signals satiety; synergistic with CCK | Gastric Chief Cell (Stomach) P-cell (Stomach) | Leptin R | CNS, GI tract, Vagal nerve |
| Peptide YY (PYY) | Inhibits food intake, triggers ileal break; acts directly on vagal afferents and crosses the blood-brain barrier (BBB) to act via NPY receptors (Y1-5 receptors), primarily through NPY2R | L-Cell (Distal SI, Proximal LI, Distal LI) | Peptide YY (PYY) Receptor | CNS, Enteric neurons |
| Somatostatin | Inhibits stomach secretion, decreases gastric emptying, slows motility | D-Cell (Stomach, Proximal SI) | Somatostatin Receptors (SSTR1, SSTR2, SSTR3, SSTR4, SSTR5) | CNS, GI tract, Immune Cells, Pancreas |
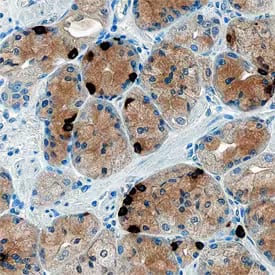
Ghrelin/Obestatin was detected in immersion fixed paraffin-embedded sections of human stomach using Sheep Anti-Human Ghrelin/Obestatin Antigen Affinity-purified Polyclonal Antibody (AF8149) at 1 µg/mL overnight at 4 °C. Tissue was stained using the Anti-Sheep HRP-DAB Cell & Tissue Staining Kit (CTS019) (brown) and counterstained with Hematoxylin (blue). Specific staining was localized to cytoplasm of gastric mucosa.
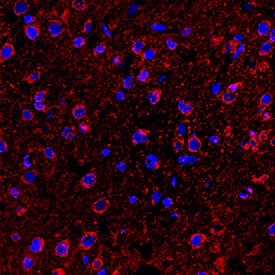
5-HT6 was detected in perfusion fixed frozen sections of rat brain (medulla) using Rabbit Anti-Human 5-HT6 Monoclonal Antibody (MAB9496) at 1.7 µg/mL overnight at 4 °C. Tissue was stained (red) using the NorthernLights™ 557-conjugated Anti-Rabbit IgG Secondary Antibody (NL004) and counterstained with DAPI (blue). Specific staining was localized to cytoplasm of neuronal cell bodies.
Microbiome Signaling
Brain Structure and the Microbiome
The gut microbiota is critical for the formation and functionality of the blood-brain barrier (BBB). The BBB serves as a selective semipermeable barrier between solutes in circulating blood and the brain. The BBB is comprised of endothelial cells, pericytes, astrocytes, oligodendrocytes, neurons, and extracellular matrix components which together function in the maintenance of CNS homeostasis.
Studies of germ free (GF) mice reveal impaired BBB function and increased permeability to macromolecules compared to normal, colonized mice. Typically, gut microbes produce short-chain fatty acids (SCFAs) which signal epithelial cells to form the BBB, increase the expression of tight-junction proteins, including claudin-5, occludin, and cytoplasmic zonula occludens-1 (zo-1)/tight junction protein 1, and decrease expression of genes encoding for myelination-related proteins.
BBB and Myelination Protein Expression in GF Mice Compared to Colonized Mice
| Protein | Expression in GF Mice compared to Colonized Mice | Function |
|---|---|---|
| Claudin-5 | Decreased | Main tight junction protein at BBB. Knock-out increases BBB permeability. |
| Occludin | Decreased | Critical protein in BBB integrity. Directly connects to ZO-1 and actin cytoskeleton. |
| ZO-1/TJP1 | Decreased | Form cytoplasmic bridge connecting tight junctions to cell cytoskeleton. |
| Egr1 | Increased | Early growth response 1 (Egr1) is involved in synaptic pruning and adult neurogenesis. |
| Mag1(AGPAT9) | Increased | Myelin associated glycoprotein 1 (Mag1(AGPAT9)) is critical for formation and maintenance of myelin sheaths. Involved in myelination during nerve regeneration. |
| Mbp | Increased | Myelin basic protein (Mbp) is expressed in oligodendrocytes following differentiation and responsible for generating compacted myelin stacks. |
| Mobp | Increased | Myelin oligodendrocyte basic protein (Mobp) has a role in compacting and stabilizing the myelin sheath. |
| Mog | Increased | Myelin oligodendrocyte glycoprotein (Mog) is a minor myelin located on outer surface of myelin sheath. MOG is an important auto-immune antigen in demyelinating disease models. |
| Olig1 | Increased | Transcription factor that promotes the formation and maturation of oligodendrocytes. |
| Plp1 | Increased | Proteolipid protein 1 (Plp1) is expressed in oligodendrocytes and Schwann cells. Plp1 has a role in myelin stabilization and compaction. |
| Sox10 | Increased | Regulates myelin expression in oligodendrocytes. |
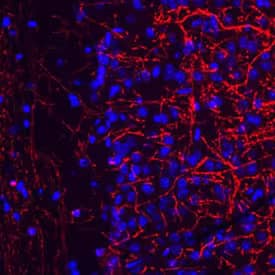
Mouse brain cryosections were stained with anti-MBP (2H9) [NBP2-22121] (1:400) and anti-mouse Alexa Fluor 555 IgG (red). Magnification 1:20. Image from verified customer review.
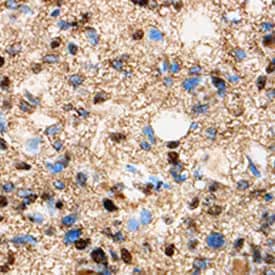
Olig1 was detected in immersion fixed paraffin-embedded sections of human glioma using 8 µg/mL Human Olig1 Monoclonal Antibody (MAB2417) overnight at 4 °C. Tissue was stained with the Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (CTS002) (brown) and counterstained with Hematoxylin (blue).
Microglia and Infection Response
Microglia are the resident immune cells of the brain. SCFAs produced by the gut microbiome have been shown to promote microglia maturation. GF mice tend to have more immature microglia that appear in a paused or surveying state.
Microglial density is increased in GF mice and they have ramified morphology, classified by smaller cell bodies and numerous, long processes. Interestingly, microglia from specific pathogen-free (SPF) mice are in an active state and have large ameboid cell bodies and retracted, stubbier processes, characteristic of maturity.
Besides morphological differences, immature and mature microglia have varying gene expression. Specifically, immature microglia have increased DNA Damage Inducible Transcript 4 (DDIT4) and Colony stimulating factor receptor 1 (CsfR1) gene expression, both of which have a role in regulating microglia cell growth, maturation, proliferation, and survival. Conversely, immature microglia have decreased Cst7, a gene associated with aging and demyelination, and Neurl3 expression compared to mature, colonized microglia.
In addition to altered microglial morphology and gene expression, GF and colonized mice have differing infection responses. Lipopolysaccharide (LPS) is a pathogen-associated molecular pattern (PAMP) that drives both GI inflammation and neuroinflammation. Bacterial challenge (such as from LPS) or viral infection causes microglia in the brain to transition from a surveying state to an active state and release pro-inflammatory cytokines.
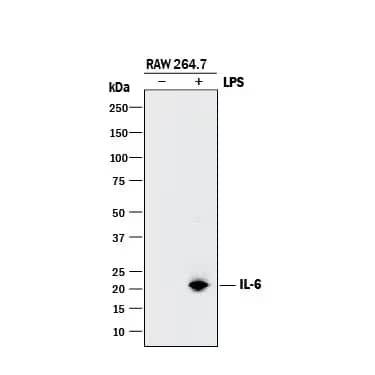
Western blot shows lysates of RAW 264.7 mouse monocyte/macrophage cell line untreated (-) or treated (+) with LPS. PVDF membrane was probed with 0.5 µg/mL of Goat Anti-Mouse IL 6 Antigen Affinity-purified Polyclonal Antibody (AF-406-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (HAF017). A specific band was detected for IL 6 at approximately 22 kDa (as indicated).
Immature (GF) microglia display an inefficient infection response compared to mature (colonized) microglia with decreased IL-1β, IL-6, TNF, and Nox2 production.
Explore Altered Infection Response in GF vs Colonized Microglia Targets
Zheng et al examined the effects of a drug with known neuroprotective effects in many neurodegenerative and neuroinflammatory diseases on spinal neuropathic pain. The researchers found that spinal neuroinflammation could be reduced by suppressing cytokine IL-1β (NB600-633) production, detected in Western Blot, indicating that the drug-induced reduction of pain was caused by inhibition of the microglial IL-1β pathway. Overall, the study highlights that microglia are one of the key players underlying neuropathic pain and therefore may be a target for treatment from CNS-related pain.

Recombinant Human CCL2/MCP 1 (279-MC) chemoattracts the BaF3 mouse pro B cell line transfected with human CCR2A in a dose-dependent manner (orange line). The amount of cells that migrated through to the lower chemotaxis chamber was measured by Resazurin (AR002). Chemotaxis elicited by Recombinant Human CCL2/MCP 1 (75 ng/mL) is neutralized (green line) by increasing concentrations of Human CCL2/MCP 1 Monoclonal Antibody (MAB679). The ND50 is typically 0.5-2.0 µg/mL.
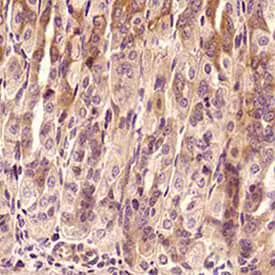
Analysis of a FFPE tissue section of mouse intestine using TNF-alpha antibody (NBP1-19532) at 1:300 dilution. The binding of this primary antibody to TNF-α protein in the section was detected using HRP-labeled secondary antibody and DAB reagent, and nuclei of cells were counterstained using Hematoxylin. This TNF-α antibody generated an expected diffused immunostaining of the protein in the tested tissue. Staining was primarily observed in the epithelial cells and some cells showed membrane positivity also.
Neurodegenerative Diseases
Neurological diseases and disorders do not solely originate in the brain, but rather are influenced by a variety of conditions and peripheral factors. Neuroinflammation, disrupted metabolism, and oxidative stress are common features of neurodegeneration and advancement of disease. Microglia respond to the PAMPS and damage-associated molecular patterns (DAMPs) associated with infection or disease states by producing reactive oxygen species (ROS), which further contribute to neurodegenerative pathologies such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Amyotrophic lateral sclerosis (ALS).
In a study by Lee et al, human umbilical cord blood cells and plasma were tested as a treatment for PD. The researchers showed reduction in both immune cell activation marker MHC Class II RT1B Antibody (NB100-65541) and proinflammatory cytokine production along with decreased inflammatory microbiota in the gut, highlighting a possible role of gut dysbiosis in neurodegeneration.
Bio-Techne offers a variety of pre-fixed, ready to use slides from both normal and diseased brain tissue including AD, PD, Multiple Sclerosis, Depression, Dementia, and Progressive Supranuclear Palsy.
Hematoxylin & Eosin Stain: Brain Tissue Slides (Parkinson's) [NBP2-77716] - Tissue: Human Brain, Pathology: Parkinson's Disease
Resources and Support
Gut-Brain Axis Resources
Gut-Brain Axis Related Research
Organoid Resources
Abdel-Haq, R., Schlachetzki, J., Glass, C. K., & Mazmanian, S. K. (2019). Microbiome-microglia connections via the gut-brain axis. The Journal of experimental medicine. https://doi.org/10.1084/jem.20180794
Bisht, K., Sharma, K., & Tremblay, M. È. (2018). Chronic stress as a risk factor for Alzheimer's disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiology of stress. https://doi.org/10.1016/j.ynstr.2018.05.003
Buford T. W. (2017). (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. https://doi.org/10.1186/s40168-017-0296-0
Chukwurah, E., Osmundsen, A., Davis, S. W., & Lizarraga, S. B. (2019). All Together Now: Modeling the Interaction of Neural With Non-neural Systems Using Organoid Models. Frontiers in neuroscience. https://doi.org/10.3389/fnins.2019.00582
Cryan, J. F., O'Riordan, K. J., Cowan, C., et al. The Microbiota-Gut-Brain Axis. Physiological reviews. https://doi.org/10.1152/physrev.00018.2018
Morelli, E., Morillas, E., O'Connor, R., … Dinan, T. G. (2019). The Microbiota-Gut-Brain Axis. Physiological reviews. https://doi.org/10.1152/physrev.00018.2018
Dinan, T. G., & Cryan, J. F. (2017). The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterology clinics of North America. https://doi.org/10.1016/j.gtc.2016.09.007
Fukui, H., Xu, X., & Miwa, H. (2018). Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. Journal of neurogastroenterology and motility. https://doi.org/10.5056/jnm18071
Kadry, H., Noorani, B., & Cucullo, L. (2020). A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and barriers of the CNS. https://doi.org/10.1186/s12987-020-00230-3
Kaelberer, M. M., Rupprecht, L. E., Liu, W. W., Weng, P., & Bohórquez, D. V. (2020). Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annual review of neuroscience. https://doi.org/10.1146/annurev-neuro-091619-022657
Kaelberer, M. M., Buchanan, K. L., Klein, M. E., Barth, B. B., Montoya, M. M., Shen, X., & Bohórquez, D. V. (2018). A gut-brain neural circuit for nutrient sensory transduction. Science. https://doi.org/10.1126/science.aat5236
Latorre, R., Sternini, C., De Giorgio, R., & Greenwood-Van Meerveld, B. (2016). Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. https://doi.org/10.1111/nmo.12754
Lee, D. H., & Linker, R. A. (2012). The role of myelin oligodendrocyte glycoprotein in autoimmune demyelination: a target for multiple sclerosis therapy?. Expert opinion on therapeutic targets. https://doi.org/10.1517/14728222.2012.677438
Lee, J. Y., Tuazon, J. P., Corey, S., Bonsack, B., Acosta, S., Ehrhart, J., Sanberg, P. R., & Borlongan, C. V. (2019). A Gutsy Move for Cell-Based Regenerative Medicine in Parkinson's Disease: Targeting the Gut Microbiome to Sequester Inflammation and Neurotoxicity. Stem cell reviews and reports. https://doi.org/10.1007/s12015-019-09906-2
Liddle R. A. (2019). Neuropods. Cellular and molecular gastroenterology and hepatology. https://doi.org/10.1016/j.jcmgh.2019.01.006
Mosher, K. I., & Wyss-Coray, T. (2015). Go with your gut: microbiota meet microglia. Nature neuroscience. https://doi.org/10.1038/nn.4051
Rao, M., & Gershon, M. D. (2016). The bowel and beyond: the enteric nervous system in neurological disorders. Nature reviews. Gastroenterology & hepatology. https://doi.org/10.1038/nrgastro.2016.107
Sampson, T. R., & Mazmanian, S. K. (2015). Control of brain development, function, and behavior by the microbiome. Cell host & microbe. https://doi.org/10.1016/j.chom.2015.04.011
Sharon, G., Sampson, T. R., Geschwind, D. H., & Mazmanian, S. K. (2016). The Central Nervous System and the Gut Microbiome. Cell. https://doi.org/10.1016/j.cell.2016.10.027
Simpson, D., & Oliver, P. L. (2020). ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel, Switzerland). https://doi.org/10.3390/antiox9080743
Stadelmann, C., Timmler, S., Barrantes-Freer, A., & Simons, M. (2019). Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiological reviews. https://doi.org/10.1152/physrev.00031.2018
Veremeyko, T., Yung, A., Dukhinova, M., Strekalova, T., & Ponomarev, E. D. (2019). The Role of Neuronal Factors in the Epigenetic Reprogramming of Microglia in the Normal and Diseased Central Nervous System. Frontiers in cellular neuroscience. https://doi.org/10.3389/fncel.2019.00453
Xu, L., He, D., & Bai, Y. (2016). Microglia-Mediated Inflammation and Neurodegenerative Disease. Molecular neurobiology. https://doi.org/10.1007/s12035-015-9593-4
Zheng, S. H., Yan, C. Y., Duan, N., Wang, W., & Mei, X. P. (2019). Penehyclidine hydrochloride suppressed peripheral nerve injury-induced neuropathic pain by inhibiting microglial MAPK/p-p38/IL-1β pathway activation. Molecular pain, 15, 1744806919858260. https://doi.org/10.1177/1744806919858260

