Hypoxia Signaling
The cellular response to hypoxia is predominantly shaped by HIF signaling. HIFs, primarily HIF-1, HIF-2 and to a less-defined extent HIF-3, control the expression of hundreds of genes involved in key cellular processes that facilitate adaptation to low-oxygenation.
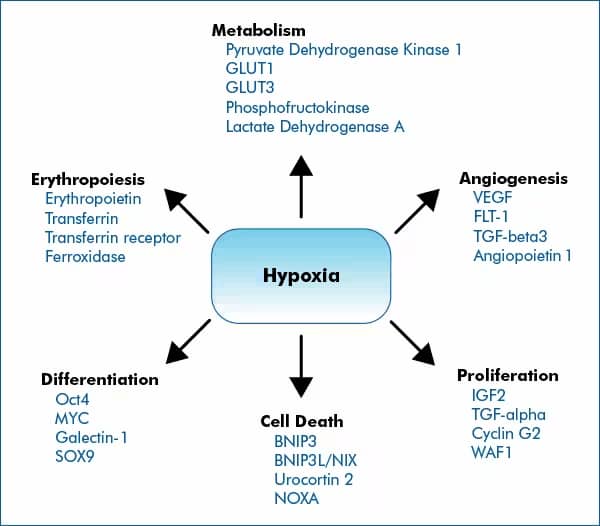
This list is not all inclusive of genes regulated by HIF proteins, but include representative genes
HIF-Dependent Transcriptional Regulation
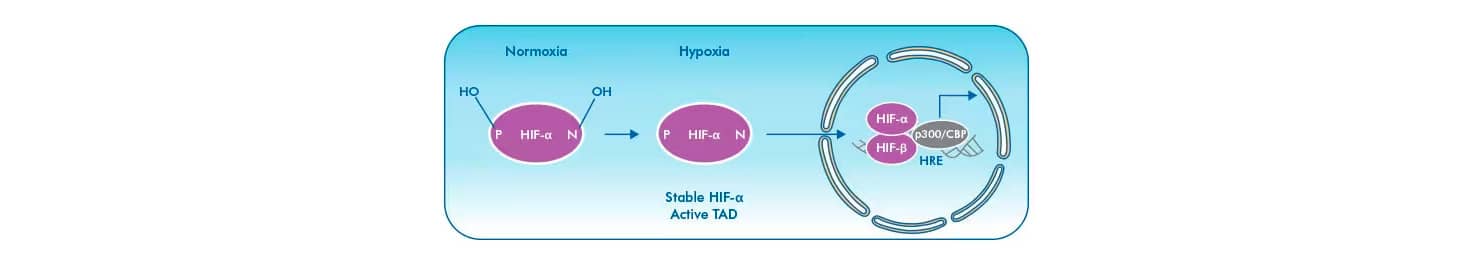
Transcriptional activity of HIF proteins is sensitive to oxygen via modulation by Factor Inhibiting HIF (FIH). Hydroxylation of HIF-1α’s asparagine-803 (Asn-803) and HIF-2α’s Asn-851 by FIH under normoxic conditions prevents association with the coactivators p300/CBP and inhibits transcriptional activation of target genes. Hypoxia inactivates FIH, preventing hydroxylation of HIFs and allowing their association with coactivators. FIH enzymes more efficiently catalyze the hydroxylation of the ASN residue in HIF-1α, when compared to HIF-2α.
NF-κB Signaling
NF-κB is a main regulator of inflammatory signaling, orchestrating the induction of proinflammatory proteins including adhesion molecules, cytokines and chemokines. NF-κB signaling is activated via three mechanisms including canonical (dependent on inhibitor of κB kinase -IKK beta), non-canonical pathways (dependent on IKK-alpha) and atypical (IKK-dependent or -independent) pathways. Hypoxia activates NF-κB via the canonical pathway leading to the induction or repression of various target genes.
| Hypoxia/NF-kB Regulated Genes | |
|---|---|
| Cytokines/Chemokines | TNF-α, IL-1β, IL-6, IL-8 |
| Enzymes | COX-2, iNOS |
| Adhesion Proteins | ICAM-1, VCAM-1 |
| Transcription Factors | HIF-1α |
| Immunoreceptors | TLR-3 |
A recent proposed mechanism supports that induction of NF-κB in hypoxia is mediated by the activation of TAK1 and IKK, a process requiring the E3-ubiquitin ligase activity of XIAP.
PI 3-K/AKT SignalingThe PI 3-K/AKT pathway is involved in various cellular processes including metabolism, proliferation and survival. This pathway is commonly induced through the activation of growth factor receptors (e.g. EGFR). Stress signals, including reactive oxygen species (ROS) and hypoxia may also lead to AKT activation, preventing apoptosis. The hypoxia-dependent mechanism underscoring the activation of the PI 3-K/AKT pathway is cell specific and not clearly defined. Learn about the new link between AKT and hypoxia: Novel Insights into Hypoxia-Induced AKT Signaling
|
||||||||
Unfolded Protein Response (UPR)
Several stress conditions affect the endoplasmic reticulum’s (ER) function in the maturation of transmembrane and secreted proteins. Hypoxia results in the accumulation of misfolded proteins within the ER, triggering the unfolded protein response (UPR) signaling pathway. Overall, the molecular pathways that conform the UPR response aim at maintaining ER homeostasis by regulating protein processing, cellular-metabolism and -survival.

The exact molecular mechanisms initiated by hypoxia which trigger UPR signaling are not well understood.
Three ER-stress sensors participate in the UPR response including:
- PRK-like ER kinase (PERK)- inhibits mRNA translation
- Inositol-requiring protein 1 (IRE-1)- induces the degradation of XBP1 mRNA
- Activating transcription factor 6 (ATF6)- translocates to the Golgi for processing and becomes transcriptionally active
These integral membrane proteins become activated following ER stress due to reduced binding to BIP (chaperone immunoglobulin heavy chain-binding protein) or by interaction with misfolded proteins. Together, these pathways induce proteins which promote the proper processing of ER-localized proteins.
Learn more with UPR and ER Stress FAQs or request our Antibodies for Unfolded Protein Response (UPR) Brochure.