Microbiology Research Scope
Microbiology research encompasses the study of all microscopic entities including unicellular and multicellular life forms, and non-cellular or acellular particles. When microbes form symbiotic relationships with hosts, either both species benefit from the host-microbe interaction, classified as mutualism, or is described as commensalism where only one species benefits. Conversely in parasitism one species, the parasite, benefits at the expense of the other, the host, often resulting in an infection and/or disease.

Unicellular
Bacteria - Prokaryotic unicellular forms are defined by their lack of organized membrane-enclosed DNA or nuclei and organelles.
Protozoa- Eukaryotic unicellular organisms include free living and parasitic forms, such as malaria-causing Plasmodium species.
Acellular
Viruses - Non-cellular particles consistent of genetic material (DNA or RNA) and proteins. Viruses infect cells of multicellular and unicellular organisms which serve as hosts for their replication.
Microbial Diseases
Medical microbiology focuses on pathogenic microbes, which represent about 1% or less of all microorganisms. Microbiology as an area of research is closely intertwined with immunology, particularly in the study of host-pathogen interactions in infectious diseases. Microbial pathogen structures commonly referred to as pathogen-associated molecular patterns (PAMPs) are first recognized by cells of the innate immune system through a broad range of pattern recognition receptors (PRRs).
Microbial Virulence
Microbial pathogens induce tissue damage by their unique invasive phenotypes and virulence factors. Bacteria produce endotoxins (e.g., lipopolysaccharide or LPS) and exotoxins (e.g., Botulinum toxin and Clostridium difficile toxin A and B). LPS is a predominant component of the Gram-negative bacterial outer membrane containing a toxic lipid component, Lipid A, which triggers a generalized inflammatory response. In contrast, exotoxins are proteins produced predominantly by Gram-positive bacteria that act upon specific cellular receptors leading to cell damage.
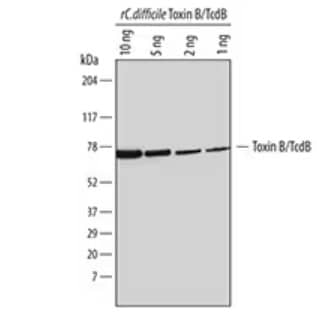
Clostridium difficile toxin B (TcdB) is one of the main exotoxins produced by C. difficile which induces cytotoxicity through the inactivation of GTP binding proteins (Rho, Rac, and Cdc42). Western blot analysis of recombinant C. difficile Toxin B/TcdB (6246-GT). PVDF membrane was probed with 1 µg/mL of Sheep Polyclonal Antibody to C. difficile Toxin B/TcdB Antigen (AF6246) followed by HRP-conjugated Anti-Sheep IgG Secondary Antibody (HAF016). A specific band was detected for Toxin B/TcdB at approximately 75 kDa.
Virus virulence determinants are encoded by both structural and non-structural proteins. Virulence factors act through different mechanisms to modulate virus-host interactions, including the regulation of viral replication, attachment, cell entry, and transmissibility. Additionally, virulence factors may modulate the host’s immune system allowing viruses to remain undetected. This is best exemplified by the immune-modulating factors produced by large DNA viruses such as poxviruses and herpes viruses which either inhibit or mimic host’s cytokines, chemokines, and proteases. Find out about SARS-CoV-2 non-structural proteins.
| Virus | Immunomodulating Protein | Immune Evasion Mechanism |
|---|---|---|
| Poxvirus-Variola | Cytokine response modifier (CRMB) | Soluble tumor necrosis factor receptor (TNFR) homologue, binds to chemokines preventing immune cell recruitment. |
| Hepatitis C Virus (HCV) | Nonstructural protein 3 (NS3) | Serine protease, targets the mitochondrial antiviral signaling protein (MAVS) to inhibit IFN-beta production. |
| Herpes Simplex Virus (HSV) |
UL36 ubiquitin-specific protease (UL36USP) ICP34.5 Kinase US3 |
Tegument protein with deubiquitinase activity, inhibits INF-beta production. Inhibits production of IFN thorough TBK1 sequestration. Serine/threonine kinase, reduces activation of type I IFN. |
Human Microbiota
The human microbiota is expansive, consisting of trillions of microorganisms inhabiting different organs and tissues throughout our bodies. By definition, the genes associated with the community of microbes (bacteria, virus, fungi and more) inhabiting symbiotically the human body, conform the microbiome. Among human organs, the gastrointestinal tract and gut mucosa represent the most populated niche for microbiota-host interactions. The gastrointestinal tract is primarily populated by Firmicutes and Bacteroidetes species. The microbiota, through interactions with the gut epithelium, is implicated in the regulation of metabolic processes and immune responses.
| Predominant phyla Bacteroidetes Firmicutes |
Health Development Digestion Immunity Metabolism |
| Minor phyla Proteobacteria Verrucomicrobia Actinobacteria Fusobacteria Cyanobacteria |
Disease Atherosclerosis Diabetes IBD Liver Disease Metabolic Syndrome Neurodegeneration Obesity |
The human gut microbiota is implicated in the modulation of a wide range of physiological processes including digestion (e.g., processing of indigestible substrates), metabolism (e.g., glucose and lipid homeostasis), immunity (e.g., pathogen exclusion), development (e.g., neuronal, epithelial and immune cells).
Microbial activity in the gut generates a wide range of metabolites including indoles, folate, secondary bile acids and neurotransmitters such as serotonin and GABA. Microbiota-derived metabolites such as short chain fatty acids (e.g., acetic, propionic and butyric acid), interact with cellular receptors in the gut epithelium inducing the release of peptides involved in glucose metabolism.
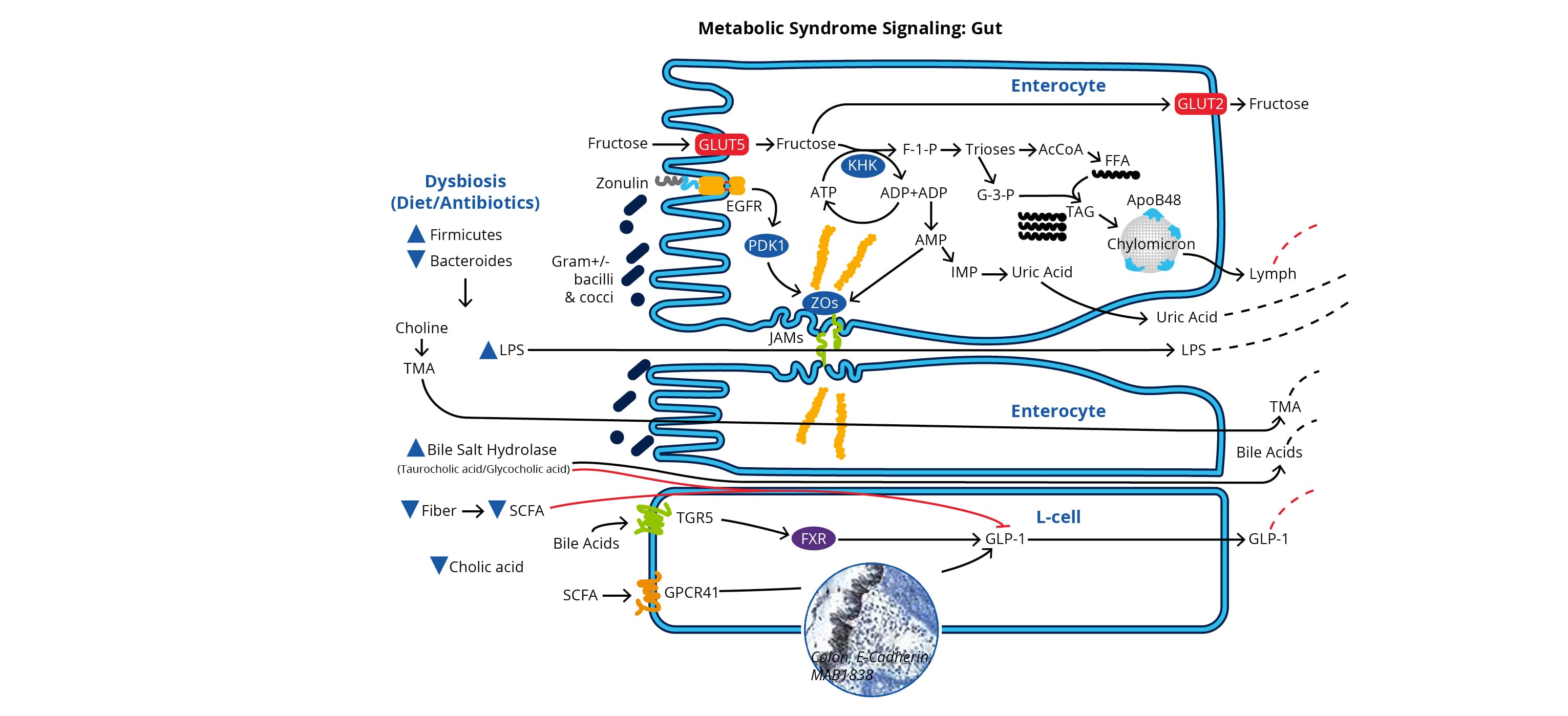
Gut microbiota dysbiosis, resulting from poor diet (high-fat and low-fiber) and/or antibiotic use, is associated with abnormal metabolite levels, increased LPS, and decreased tight junction integrity. Reduced tight junction integrity, allows LPS leakage across the gut epithelial barrier, which contributes to chronic inflammation of the liver and adipose tissue. Additionally, dysbiosis is associated with reduced levels of short chain fatty acids, which normally interact with G-protein coupled receptors such as GPR-41 in the gut epithelium to promote the release of glucagon-like peptide-1 (GLP1). Reduced release of GLP1 by enteroendocrine L cells, disrupts glucose homeostasis and promotes inflammation.
Diagram from Metabolic Syndrome Signaling Poster , published in its original form in a collaboration between Dr. Robert H. Lustig, Dr. Alejandro Gugliucci and Bio-Techne Corporation.
Rineh, A. et al. (2014). Clostridium difficile infection: Molecular pathogenesis and novel therapeutics. Expert Review of Anti-Infective Therapy. https://doi.org/10.1586/14787210.2014.866515
Alejo, A., Ruiz-Argüello, M. B., Ho, Y., Smith, V. P., Saraiva, M., & Alcami, A. (2006). A chemokine-binding domain in the tumor necrosis factor receptor from variola (smallpox) virus. Proceedings of the National Academy of Sciences of the United States of America, 103(15), 5995–6000. https://doi.org/10.1073/pnas.0510462103
Wang, B., Yao, M., Lv, L., Ling, Z., & Li, L. (2017). The Human Microbiota in Health and Disease. Engineering. https://doi.org/10.1016/J.ENG.2017.01.008
Cryan, J. (2018). 37. THE GUT MICROBIOME: A KEY REGULATOR OF NEURODEVELOPMENT AND BEHAVIOUR. Schizophrenia Bulletin. https://doi.org/10.1093/schbul/sby014.152
Dabke, K., Hendrick, G., & Devkota, S. (2019). The gut microbiome and metabolic syndrome. Journal of Clinical Investigation. https://doi.org/10.1172/JCI129194
Farhana, A., & Khan, Y. S. (2020). Biochemistry, Lipopolysaccharide. In StatPearls.
Freundt, E. C., & Lenardo, M. J. (2005). Interfering with interferons: Hepatitis C virus counters innate immunity. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.0509221102
Mogensen, T. H. (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews. https://doi.org/10.1128/CMR.00046-08
Su, C., Zhan, G., & Zheng, C. (2016). Evasion of host antiviral innate immunity by HSV-1, an update. Virology Journal, 13, 38. https://doi.org/10.1186/s12985-016-0495-5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4782282/