By Hunter Martinez
What is Immunometabolism?
It is well established that abnormal metabolic environments can be a risk factor for disease development. One characteristic example is the role of dyslipidemia (high levels of lipids) in development of atherosclerosis.1 The interplay between metabolic state and immune cell functions is an area of great interest to researchers. For instance, leptin-deficient mice are resistant to experimental autoimmune encephalomyelitis (EAE), a mouse model of the autoimmune disease multiple sclerosis. In this system, addition of exogenous leptin promotes a pathologic T cell response2 and leads to disease progression. Observations like this have led to the emergence of the field of immunometabolism. Immunometabolism refers to changes in systemic or intracellular metabolic status which can influence the phenotype and function of innate and adaptive immune cells. Type 1 diabetes (T1D) is another devastating autoimmune disorder, characterized by immune-mediated destruction of beta-islet cell of the pancreas, resulting in insufficient insulin production. Evidence exists supporting the role of metabolic perturbations of immune cells in the initiation and exacerbation of T1D.
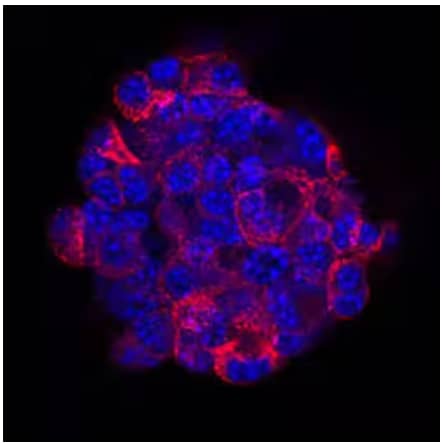
Immunocytochemical/Immunofluorescence analysis of TC-6 mouse beta cell insulinoma cell line showing insulin expression using Anti-Human/Mouse/Bovine Insulin Monoclonal Antibody (Catalog # MAB1417) followed by staining with NorthernLights™ 557-conjugated Anti-Rat IgG Secondary Antibody (Catalog # NL013) (red) and nuclei counterstaining with DAPI (blue).
Major Pathways of Intracellular Metabolism
To understand immunometabolism, it is critical to have a basic understanding of intracellular metabolic pathways. Intracellular metabolite status is informed by five major catabolic pathways: glycolysis, the pentose phosphate pathway (PPP), amino acid metabolism, fatty acid oxidation (FAO), and the tricarboxylic acid (TCA) cycle.3,4 In glycolysis, glucose is transported from the extracellular environment and catabolized into intermediates such as pyruvate and NADH for use in other metabolic pathways. The PPP allows the diversion of intermediates from the glycolytic pathway towards the production of nucleotides and amino acid precursors necessary for cell growth and proliferation.3 Additionally, the PPP generates NADPH to maintain cellular redox environment. Amino acid metabolism is regulated in part by mammalian target of rapamycin (mTOR), which can increase amino acid transport such as glutamine, necessary for protein translation and the TCA cycle. Cytosolic metabolic pathways like glycolysis and amino acid metabolism are important for providing the starting material for metabolic pathways within the mitochondria. The TCA cycle and FAO take place within the confines of the mitochondria. FAO breaks down fatty acids into acetyl-CoA to enter the TCA cycle. The TCA cycle “initiates” with acetyl-CoA, derived from pyruvate or fatty acid oxidation, and produces NADH and FADH2 which transfer electrons to the electron transport chain to support oxidative phosphorylation (OXPHOS) and ATP generation.
Role of Immunometabolism in T1D pathology
The metabolic environment of individuals with T1D is significantly altered compared to healthy counterparts. Data suggests this disrupted metabolic state influences the phenotype and function of immune cells, leading to disease progression. For example, elevated glucose levels promote M1 macrophage polarization.5 M1 macrophages are a pro-inflammatory subset of macrophages with the enhanced ability to produce cytokines such as IL-1β, TNF, IL-12, and IL-18. Furthermore, they exhibit high expression of MHC-II and co-stimulatory molecules to activate CD4+ T cells, critical mediators of T1D pathogenesis. In addition to increased M1 macrophage activity, anti-inflammatory regulatory T cells (Tregs) can become impaired under high glucose conditions.6-9 High glucose uptake is associated with reduced suppression by Tregs through modulation of co-inhibitory molecules, such as CTLA-4.8 During progression of T1D, increased glucose concentrations upregulate acyl-CoA synthase long chain 1 (ACSL1), an enzyme involved in FAO which produces many inflammatory molecules, such as PGE2 through prostaglandin-endoperoxide synthase 2 (PTGS2)/COX2 enzyme.10 PGE2 is a bioactive lipid which can facilitate tissue influx of macrophages and neutrophils.
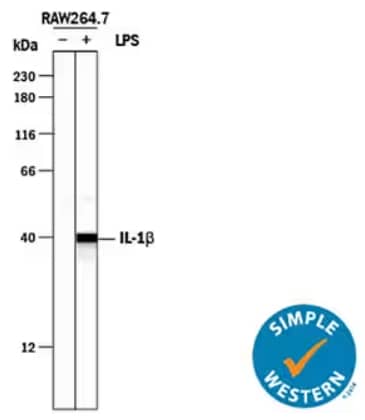
Simple Western lane view analysis of lysates from RAW 264.7 mouse monocyte/macrophage cell line either untreated (-) or treated (+) with lipopolysaccharide (LPS), probed with Goat Anti-Mouse IL-1 beta/IL-1F2 Polyclonal Antibody (Catalog # AF-401-NA) followed by HRP-conjugated Anti-Goat IgG Secondary Antibody (Catalog # HAF109). A specific band was detected at ~40 kDA. The Simple Western experiment was conducted under reducing conditions on a 12-230 kDa separation system.
Targeting Immunometabolism for Treatment of Autoimmunity
Albeit not expansive or exclusive, these five catabolic pathways influence immunometabolism of T1D. One current effort in the NOD mouse model of T1D is targeting glycolysis through use of a glucose analog, 2-deoxyglucose (2-DG).12 This analog structurally resembles glucose, but cannot proceed fully through the glycolytic pathway, resulting in poor expansion of autoreactive T cells and reduced islet infiltration. Research into other metabolic pathways are underway with the hope of providing alternative therapies to treat T1D and other autoimmune diseases.13
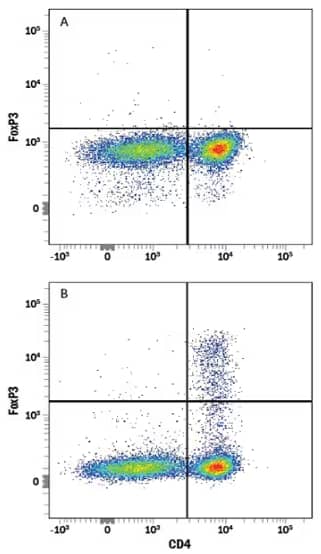
(A) Untreated or (B) Treated human peripheral blood mononuclear cells (PBMCs) stimulated with Recombinant Human TGF‑ beta 1 Protein (Catalog # 240-B) and Recombinant Human IL‑2 Protein (Catalog # 202-IL) to induce Regulatory T Cells (Tregs). Cells were stained with Rabbit Anti-FoxP3 Monoclonal Antibody (Catalog # MAB8214) followed by PE-conjugated Goat Anti-IgG Secondary Antibody (Catalog # F0110) and APC-conjugated Mouse Anti-CD4 Monoclonal Antibody (Catalog # FAB3791A). Staining with Rabbit IgG Isotype Control Antibody (Catalog # AB-105-C) was used to set quadrants.

Hunter Martinez
Stanford University School of Medicine
Hunter is a PhD graduate student studying the role of the extracellular matrix in T cell activation and function.
-
Cybulsky, M. I. et al. (1991) Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis Science (New York, N.Y.) 251:788-791.
-
Matarese, G. et al. (2001) Requirement for leptin in the induction and progression of autoimmune encephalomyelitis Journal of immunology (Baltimore, Md. : 1950) 166:5909-5916.
-
O'Neill, L. A., & Pearce, E. J. (2016) Immunometabolism governs dendritic cell and macrophage function The Journal of experimental medicine 213:15-23.
-
Makowski, L. et al. (2020) Immunometabolism: From basic mechanisms to translation Immunological reviews 295:5-14.
-
Amersfoort, J., & Kuiper, J. (2017) T cell metabolism in metabolic disease-associated autoimmunity Immunobiology 222:925-936.
-
Angelin, A. et al. (2017) Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments Cell metabolism 26:1282-1293.
-
Gerriets, V. A. et al. (2016) Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression Nature immunology 17:1459-1466.
-
Watson, M. J. et al. (2021) Metabolic support of tumour-infiltrating regulatory T cells by lactic acid Nature 591:645-651.
-
Zappasodi, R. et al. (2021) CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumours Nature 591:652-658.
-
Kanter, J. E. et al. (2012) Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl-CoA synthetase 1 Proceedings of the National Academy of Sciences of the United States of America 109:E715-E724.
-
Vignali, D. et al. (2018) Detection and Characterization of CD8+ Autoreactive Memory Stem T Cells in Patients With Type 1 Diabetes Diabetes 67:936-945.
-
Garyu, J. W. et al. (2016) Characterization of Diabetogenic CD8+ T Cells: IMMUNE THERAPY WITH METABOLIC BLOCKADE The Journal of biological chemistry 291:11230-11240.
-
Pålsson-McDermott, E. M. et al. (2020) Targeting immunometabolism as an anti-inflammatory strategy Cell research 30:300-314.