By Christina Towers, PhD
Autophagy is a catabolic process used to breakdown and recycle damaged proteins and organelles. It is a multistep process that, in its simplest form, consists of 4 steps: initiation, phagophore nucleation, elongation, and fusion – resulting in a double membrane structure called an autophagosome that, upon fusion with the acidic lysosome, facilitates the degradation of its contents. LC3B (and its family members) are incorporated into the elongating autophagophore and are necessary for efficient autophagosome formation and autophagic degradation. During lysosomal fusion, LC3B is itself degraded. For these reasons, LC3B protein levels are often used to measure autophagy function and, when used in combination with lysosomal inhibitors, can quantify autophagic flux.
LC3/GABARAP Family
The yeast homologue was first identified as Atg8. There are at least 7 Atg8 orthologues in mammals that include microtubule-associated protein 1 light chain 3 alpha, beta and gamma – LC3A, LC3B, and LC3C respectively, as well as GABA type A receptor-associated protein (GABARAP), GABARAP-Like1, and GABARAP-Like21. Of these, LC3B is the most common and best understood – and the only one accepted in the field as an accurate readout of autophagic flux.
Despite each of these proteins coming from separate transcripts with varying genomic locations, they all have high sequence similarity. Importantly, they share a conserved C-terminus glycine that is cleaved by mammalian protease, ATG4 family members, to create a soluble form, or LC3-I.
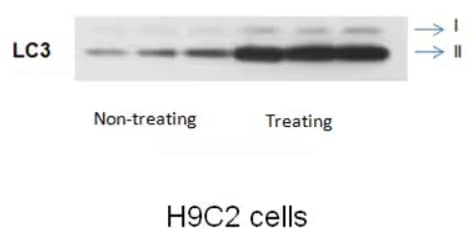
Western Blot Analysis: rabbit polyclonal anti-LC3B antibody [NB100-2220] on rat embryonic cardiomyocyte H9C2 cell line.
LC3-I interacts with the E1-like enzyme ATG7 and other aspects of the conjugation machinery, including ATG5-ATG12, in order to conjugate phosphatidylethanolamine (PE) to LC3-I to create the insoluble LC3-PE, also known as the functional LC3-II protein2. When analyzed in combination with a lysosomal inhibitor such as bafilomycin-A1 or chloroquine, turnover of the LC3-II form is the ultimate readout for autophagic flux.
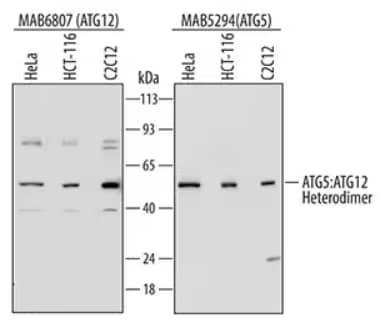
Western Blot: lysates of HeLa human cervical epithelial carcinoma cell line, HCT 116 human colorectal carcinoma cell line, and C2C12 mouse myoblast cell line. Polyvinylidene difluoride (PVDF) membrane (left) was probed with 0.1 µg/mL of Mouse Anti-Human ATG12 Monoclonal Antibody [MAB6807] followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody [HAF007]. For additional reference, PVDF membrane (right) was probed with 0.5 µg/mL of Mouse Anti-Human/Mouse/Rat ATG5 Monoclonal Antibody [MAB5294] followed by HRP-conjugated Anti-Mouse IgG Secondary Antibody [HAF007]. A specific band was detected for the ATG5:ATG12 heterodimer at approximately 60 kDa (as indicated). This experiment was conducted under reducing conditions and using Immunoblot Buffer Group 2.
LC3A, B and C Functions
Based on genetic knock out and rescue experiments, LC3A, LC3B, and LC3C are thought to function at earlier stages in the autophagy pathway and the GABARAP proteins at later steps3. All 3 of the LC3 family members are believed to have very similar functions; however, much is still unknown about their different expression patterns, and even less about their autophagy-independent functions. One study used confocal microscopy to show that LC3A and LC3B do not co-localize in a single cell, suggesting that any given autophagosome does not contain multiple isoforms such that only one is necessary at a time for a functional autophagosome to form4. Moreover, this study also showed that LC3A appears to localize to the peri-nuclear region of the cell, whereas LC3B is more evenly distributed throughout the cytoplasm. There is still much to learn about these different isoforms and their roles both inside and out of the autophagy pathway.

Christina Towers, PhD
University of Colorado (AMC)
Dr. Towers studies the roles of autophagy, apoptosis and cell death in cancer.
-
Schaaf, M. et al. (2016) LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J 30:3961-3978.
-
Towers, C.G. et al. (2016) Therapeutic Targeting of Autophagy. EBioMedicine.
-
Weidberg, H. et al. (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29:1792-1802.
-
Koukourakis, M.I. et al. (2015) Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS One