TcBuster™: Frequently Asked Questions
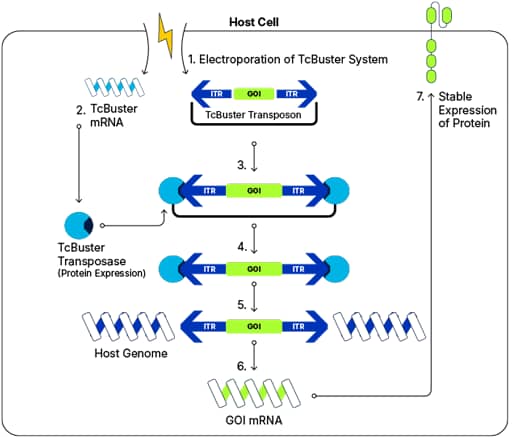
TcBuster is a non-viral gene editing system for stable gene transfer in many cell types and is based on DNA transposition.
- The TcBuster reagents, comprised of the mRNA transposase and DNA transposon, are electroporated into the cell.
- Once inside the cell, the TcBuster mRNA is translated to the transposase enzyme.
- The transposase binds to specific sites known as inverted terminal repeats (ITRs) which flank the genes of interest (GOIs) on the DNA transposon.
- TcBuster excises the DNA cargo from the transposon.
- TcBuster transposase inserts the DNA cargo randomly into the host cell genome at AT nucleotide sites.
- The DNA cargo is transcribed into mRNA.
- It is then translated into protein for stable gene expression.
Gene transfer is considered stable if the DNA is integrated into the host cell genome. We have shown that TcBuster can edit CHO cells, and that the edited cells express the integrated transgene over an extended period (60 doublings).
The TcBuster system requires these elements:
- TcBuster-M mRNA transposase
- TcBuster compatible DNA transposon (plasmid)
- An electroporation platform, such as the Lonza 4D-Nucleofector®, ThermoFisher Neon™/Neon NxT, or MaxCyte®
Research grade TcBuster-M transposase mRNA is available on the website in two pack sizes or a GMP option:
- TCB-001.1-100: For initial evaluation
- TCB-001.1-500: For larger scale work
- TCB-001.1-GMP: For pre-IND process development and ex vivo clinical applications
Research-grade experimental control DNA transposons are also available:
- TCBP001-100: EF1a-CD19CAR-DHFR-eGFP plasmid transposon
- TCBP002-100: Insert-On eGFP plasmid transposon
- For custom DNA transposons or other controls, please refer to the molecular guide or email the technical support team at techsupport@bio-techne.com
While the system is compatible with any electroporation platform, Bio-Techne has the most experience and knowledge with the Lonza Nucleofector, ThermoFisher Neon, and MaxCyte machines. All three of these platforms have a GMP-certified machine option. See our protocols here.
TcBuster-M refers to a hyperactive version of the wildtype TcBuster transposase enzyme. Hyperactive refers to the fact that the enzyme has been engineered to have increased enzymatic activity over the wildtype version of the enzyme. This increases the efficiency of gene transfer to rates that are better than or equal to alternate gene engineering systems. TcBuster-M is the only version of TcBuster transposase available for purchase. Please read a recent publication describing the generation of the hyperactive TcBuster enzyme.
TcBuster-M transposase products are solely offered as mRNA to minimize the possibility of extended editing events, which could occur if the transposase was delivered as a plasmid. Additionally, delivering the transposase as an mRNA decreases DNA-mediated toxicity.
TcBuster-M transposase is not available as a protein, as transposases are difficult to manufacture at the high concentrations and purity needed for use.
The TcBuster system is a versatile genome editing tool and has applications in a wide variety of cell types. TcBuster can be useful in:
- Cell therapy applications for genome modification in cells such as T, NK, and iPSC
- Generating stable producer cell lines, such as CHO or HEK293
- Cell line development for disease models and drug screening
Why is it recommended to use the Nanoplasmid™ (or similar technology) for cell therapy applications?
T cells, NK cells and other cell types can be affected by DNA-mediated toxicity when high concentrations of plasmid DNA are used. Minimizing the plasmid backbone by using the Nanoplasmid, or similar technology, allows for a higher ratio of GOI sequence per µg of plasmid delivered. The Nanoplasmid can be delivered to cells at a lower concentration than a traditional plasmid with the same gene editing efficiency. Improved viability and cell growth are observed after editing with lower concentrations of DNA.
Regulatory bodies, including the FDA, are recommending plasmids used in genome editing for clinical applications do NOT contain antibiotic resistance genes for safety purposes. The Nanoplasmid does not contain a bacterial antibiotic resistance gene and is a good option for a TcBuster transposon.
Yes, other non-viral genome engineering technologies can be used in combination during a single electroporation step.
There are theoretically no maximum limitations to cargo size. We typically see > 40% gene editing efficiency with most constructs for CAR or TCR cell therapy applications. However, as construct size and complexity increase, transposition rates may decline.
Yes, both T and NK cells are > 90% viable at time of harvest and undergo robust expansion in a G-Rex with multiple media types.
Yes, with TcBuster you can use a single transposon to deliver multiple genes (i.e. multicistronic) at once with equivalent or often higher editing efficiencies than viral vectors. While viral vectors can carry multiple genes, packaging limitations lead to inconsistent and low yields. The TcBuster transposon system is ideal for transferring large and complex DNA cargos.
The TcBuster insertional profile is more random when compared to lentiviral vectors. Lentivirus insertions preferentially integrate into transcriptional units with greater than 75% of insertions in exons and transcripts. TcBuster shows 20-30% reduction in exon and transcript insertions. Thus, the TcBuster system has a decreased likelihood of disrupting active genes. Check out the Lentiviral vs. Non-Viral Delivery for CAR-T Manufacturing App Note to learn more.
For lentiviral vectors the FDA guidelines state a VCN of ≤ 5 copies per genome equivalent. While no official guidelines exist for DNA transposon systems, we’ve generated protocols that aim for a low copy number (≤ 8) on multiple platforms for both T and NK cells, while maintaining high gene transfer efficiency.
Most transposition happens within 48 hours after electroporation. Using qRT-PCR we can detect TcBuster-M mRNA. The TcBuster-M mRNA is degraded over time and is undetectable 7 days after electroporation. An antibody has been generated to detect the TcBuster-M transposase protein. Within 2-3 days after electroporation, TcBuster-M transposase can no longer be detected in the cell.
This suggests that editing with the TcBuster system is transient and does not persist over an extended period.
Just like Sleeping Beauty and PiggyBac, TcBuster is a DNA transposon system. When compared side by side, TcBuster compares favorably to these other transposon systems in editing efficiency and copy number for cell therapy applications. Additionally, TcBuster is the only non-exclusive, commercially available system.
We have partnered with Aldevron to supply custom research-grade DNA transposons to our TcBuster customers. Our TcBuster Nanoplasmid backbone with compatible ITRs is banked at Aldevron which allows for seamless delivery of custom plasmids. We will connect you with our contact at Aldevron to perform the gene synthesis, cloning, and manufacturing. Our scientific support team at techsupport@bio-techne.com is available to help you navigate this process and answer your questions.
Reference our guide to creating a custom DNA transposon for the TcBuster system. The easiest way to get started is to email nanoplasmids@aldevron.com and let them know you would like to generate a custom TcBuster transposon.
The cost of ordering a transposon from a vendor depends upon the sequence and size of the construct. A custom DNA transposon will typically cost a few thousand dollars for a 1 mg batch, with costs per mg decreasing as larger batches of material is ordered.
The timeline for generating a custom DNA transposon averages 7-10 weeks.
For testing the system in your hands, for internal research purposes, no agreement (i.e. MTA) is needed. A non-exclusive license is needed for any commercial activities involving the TcBuster system. A non-exclusive license is also required to access GMP TcBuster materials.
Please contact our licensing team at tcbsupport@bio-techne.com for more information.
TcBuster-M mRNA transposase is considered an ancillary material. We are drafting a DMF for the TcBuster-M mRNA to best support clinically oriented customers. Letters of authorization can be generated for those outside the US.
To date, the TcBuster-M mRNA has been approved in 3 INDs for Luminary Therapeutics. Learn more here.
GMP TcBuster mRNA transposase is available to order exclusively from Bio-Techne.
To access GMP TcBuster materials, a non-exclusive license is required. Please contact our licensing team at tcbsupport@bio-techne.com for more information.
We no longer offer genome engineering or cell line development services. With the TcBuster system, we’ve moved to putting the power of genome editing directly into your lab.