TIM-3: An Emerging Immuno-Oncology Target
TIM-3 is an Inhibitory Receptor that Binds Gal-9, CEACAM-1, and HMGB1
T cell immunoglobulin- and mucin-domain-containing 3 (TIM-3), also known as Hepatitis A virus cellular receptor 2 (HAVCR2), is a member of the TIM family of proteins, which consists of TIM-1, TIM-3, and TIM-4 in humans and TIM-1-8 in mice.1 TIM family proteins are type I transmembrane glycoproteins with an extracellular domain consisting of an N terminal IgV-like domain, a mucin-like domain and a stalk domain, with potential O- and N-linked glycosylation sites, respectively. The extracellular domain is followed by a transmembrane segment, and with the exception of TIM-4, a cytoplasmic tail with a conserved region of five tyrosine residues that are critical for downstream signaling.2,3 TIM-3 is expressed on IFN-gamma-producing CD8+ T cells and Th1 cells, and at lower levels on Th17 cells, and has been shown to negatively regulate T cell proliferation and cytokine production.1-6 Additionally, TIM-3 is expressed on subsets of regulatory T cells, dendritic cells (DCs), macrophages, monocytes, and mast cells.7, 8 Similar to PD-1, it is also highly expressed on exhausted T cells and natural killer cells in chronic infections and in cancer.9-12
The IgV domain of the TIM-3 protein has been shown to bind to four different ligands, including galectin-9, phosphatidylserine (PtdSer), high mobility group protein B1 (HMGB1), and CEACAM-1.13-18 Galectin-9, the first identified TIM-3 ligand, was found to bind to the carbohydrate motif in the IgV domain of TIM-3 and induce Th1 cell apoptosis.13 In contrast to Galectin-9, which has a distinct binding site on the TIM-3 protein, the other TIM-3 ligands have overlapping binding sites in the FG-CC’ loop region in the IgV domain of the TIM-3 protein.3 PtdSer is a molecule that is exposed on the surface of apoptotic cells that binds not only to TIM-3, but also to TIM-1 and TIM-4, and has been implicated in macrophage and dendritic cell-mediated uptake of apoptotic cells and antigen cross-presentation.19 HMGB1 is a damage-associated molecule that has also been shown to bind to the TIM-3 protein.16 HMGB1 is released by stressed or dying cells and plays a role in transporting nucleic acids into endosomes, which is necessary to promote activation of the innate immune response against pathogens and tumors. Binding of HMGB1 to TIM-3 interferes with this process and therefore inhibits innate immunity and the anti-tumor immune response. Finally, the TIM-3 protein interacts with CEACAM-1 and the cis interaction between these two proteins has been shown to be required for TIM-3 glycosylation, stability, and its ability to inhibit T cell functions.3,17 The CEACAM-1 and TIM-3 proteins can also interact in trans to negatively regulate T cell responses.3
TIM-3 is highly expressed on CD8+ tumor-infiltrating lymphocytes (TILs) and on CD4+ regulatory T cells in multiple forms of cancer.3, 7, 11, 20, 21 All TIM-3+ CD8+ TILs co-express PD-1 and these T cells represent the most dysfunctional population of CD8+ TILs in both mouse tumor models and in many different types of human cancer.22 While blockade of TIM-3 alone was shown to have variable effects in different mouse tumor models, blockade of both TIM-3 and PD-1 was found to synergistically reduce tumor growth.9, 22-24 The positive anti-tumor effects observed by combined TIM-3/PD-1 blockade was attributed to both restoring the function of exhausted CD8+ TILs, including tumor antigen-specific T cell proliferation and cytokine production, as well as inhibiting the immunosuppressive functions of TIM-3+ regulatory T cells. Additionally, combined TIM-3/PD-1 blockade may improve anti-tumor immune responses by restoring the activity of exhausted natural killer cells, inhibiting the Galectin-9-TIM-3-mediated expansion of immunosuppressive myeloid-derived suppressor cells (MDSCs), and/or preventing TIM-3 on intratumoral DCs from interacting with HMGB1, thereby restoring activation of the innate immune response by tumor cell-derived nucleic acids. 25,26 Therefore, combination therapies targeting TIM-3 and PD-1 or CTLA-4, or those which target TIM-3 in combination with agonist antibodies for T cell co-stimulatory receptors warrant further exploration.
Bio-Techne's R&D SystemsTM brand offers bioactive recombinant proteins for TIM-3, Galectin-9, CEACAM-1, and HMGB1, along with fluorochrome-conjugated antibodies for detecting these molecules to further our understanding of the immunosuppressive functions of TIM-3 and its ligands.
TIM-3 and TIM-3 Ligands - Products by Molecule
How Does Tim-3 Inhibit Immune Cell Functions?
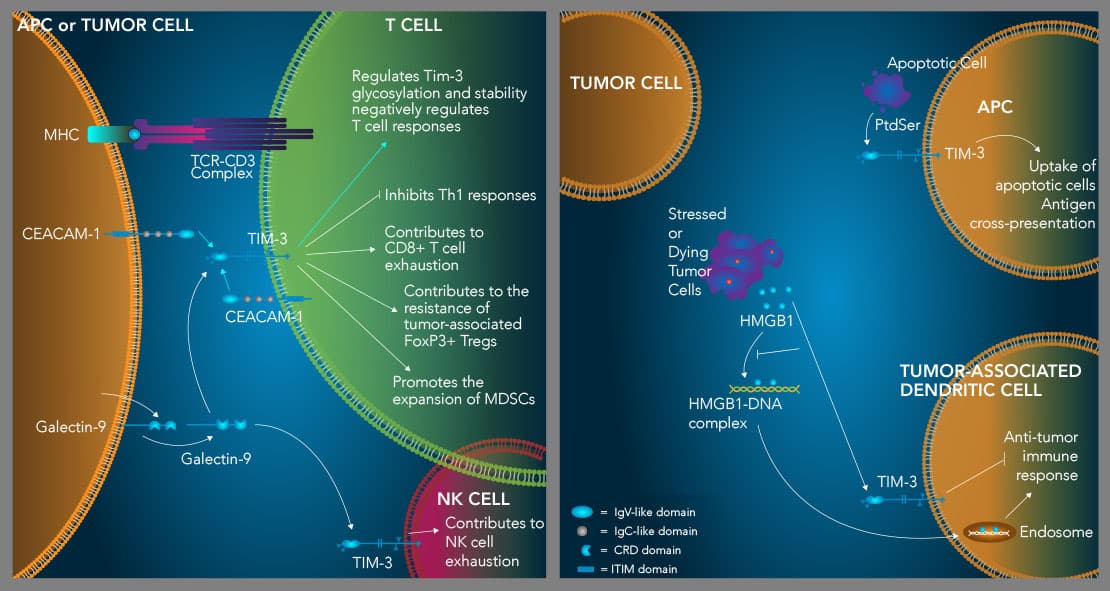
TIM-3 suppresses Th1 immune responses, contributes to T cell and NK cell exhaustion, and enhances the suppressive activity of Tregs. TIM-3 is an inhibitory receptor expressed on multiple hematopoietic cell types. High level expression of TIM-3 on CD8+ T cells and natural killer cells is associated with an exhausted phenotype, while TIM-3 expression on tumor-associated FoxP3+ regulatory T cells (Tregs) marks a subset of Tregs with enhanced suppressor functions and increased resiliency. TIM-3 binds to four different ligands including Galectin-9 (Gal-9), CEACAM-1, HMGB1, and phosphatidylserine (PtdSer). TIM-3 is expressed on Th1 cells and binding of galectin-9 to TIM-3 on these cells induces Th1 cell apoptosis. IFN-gamma produced by Th1 cells up-regulates the expression of galectin-9, which promotes the expansion of myeloid-derived suppressor cells (MDSCs). MDSCs produce increased levels of galectin-9, further driving Th1 cell apoptosis and CD8+ T cell exhaustion. TIM-3 also binds to CEACAM-1, which is required for TIM-3 glycosylation, stability, and its ability to negatively regulate T cell responses (left). Additionally, TIM-3 interacts with the damage-associated molecule, HMGB1, which inhibits the ability of HMGB1 to transport tumor-derived nucleic acids into endosomes in tumor-associated dendritic cells to initiate anti-tumor immune responses (right). Finally, TIM-3 binds to PtdSer on apoptotic cells and mediates apoptotic cell uptake and antigen cross-presentation by dendritic cells (right). How this interaction may affect TIM-3+ T cells is currently unknown.
Analysis of the Binding of R&D Systems Recombinant Human Gal-9 and TIM-3 Proteins
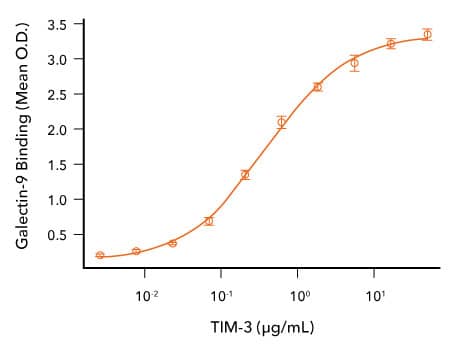
TIM-3 Binds to Galectin-9. Recombinant Human Galectin-9 (R&D Systems, Catalog # 2045-GA) was immobilized at 1 μg/mL, 100 μL/well, and the indicated concentrations of Recombinant Human TIM-3 (R&D Systems, Catalog # 10241-TI) were added. Recombinant Human TIM-3 bound with an ED50 of 0.065-0.65 μg/mL.
Assessment of the Purity of R&D Systems Recombinant Human TIM-3 Protein
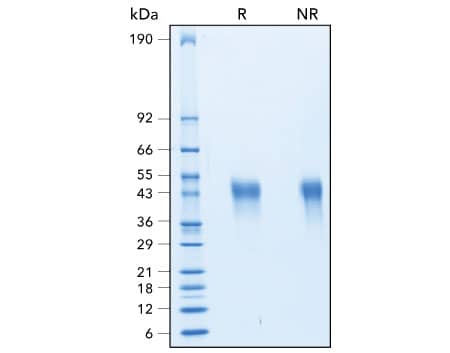
Assessment of the Purity of Recombinant Human TIM-3 by SDS-PAGE. The purity of Recombinant Human TIM-3 (R&D Systems, Catalog # 10241-TI) was assessed by SDS-PAGE analysis under reducing (R) and non-reducing (NR) conditions and visualized by Coomassie® Blue staining.
Analysis of the Bioactivity of R&D Systems Recombinant Cynomolgus Monkey TIM-3 Protein
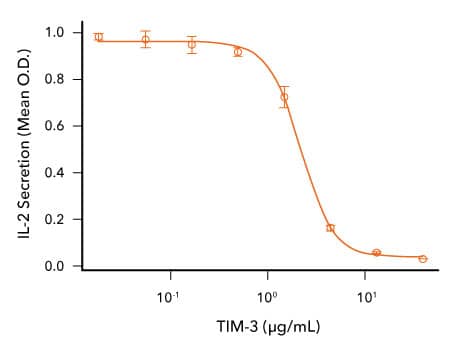
TIM-3 Inhibits Anti-CD3-induced IL-2 Secretion by Human T Cells. Human T cells were incubated with an immobilized Mouse Anti-Human CD3 epsilon Monoclonal Antibody (R&D Systems, Catalog # MAB100; 1μg/mL) and the indicated concentrations of Recombinant Cynomolgus Monkey TIM-3 (R&D Systems, Catalog # 7914-TM). IL-2 secretion was measured in cell culture supernatants using the Human IL-2 QuantikineTM ELISA Kit (R&D Systems, Catalog # D2050). The ED50 for this effect is typically 0.5-3 μg/mL.
-
Freeman, G.J. et al. (2010) TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 235:172. PMID: 20536563.
-
Monney, L. et al. (2002) Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536. PMID: 11823861.
-
Das, M. et al. (2017) Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 276:97. PMID: 28258697.
-
Sanchez-Fueyo, A. et al. (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093. PMID: 14556005.
-
Hastings, W.D. et al. (2009) Tim-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39:2492. PMID: 19676072.
-
Anderson, A.C. et al. (2016) Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 44:989. PMID: 27192565.
-
Anderson, A.C. et al. (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318:1141. PMID: 18006747.
-
Zhao, H. et al. (2017) Tregs: where we are and what comes next? Front. Immunol. 8:1578. PMID: 29225597.
-
Sakuishi, K. et al. (2013) Tim3+FoxP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2:23849. PMID: 23734331.
-
Jones, R.B. et al. (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763. PMID: 19001139.
-
Sakuishi, K. et al. (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207:2187. PMID: 20819927.
-
Gallois, A. et al. (2014) Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology 3:946365. PMID: 25964857.
-
Zhu, C. et al. (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245. PMID: 16286920.
-
Cao, E. et al. (2007) T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity 26:311. PMID: 17363302.
-
Bu, X. et al. (2010) T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. J. Immunol. 184:1918. PMID: 20083673.
-
Chiba, S. et al. (2012) Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 13:832. PMID: 22842346.
-
Huang, Y.H. et al. (2015) CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517:386. PMID: 25363763.
-
Sabatos-Peyton, C.A. et al. (2017) Blockade of Tim-3 to phosphatidylserine and CEACAM1 is a shared feature of anti-Tim-3 antibodies that have functional efficacy. Oncoimmunology 7:1385690. PMID: 29308307.
-
Nakayama, M. et al. (2009) Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood 113:3821. PMID: 19224762.
-
Gao, X. et al. (2012) TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One 7:30676. PMID: 22363469.
-
Sun, H. & C. Sun (2019) The rise of NK cell checkpoints as promising therapeutic targets in cancer immunotherapy. Front. Immunol. 10:2354. PMID: 31681269.
-
Anderson, A.C. (2014) Tim-3: An emerging target in the cancer immunotherapy landscape. Cancer Immunol. Res. 2:393. PMID: 24795351.
-
Ngiow, S.F. et al. (2011) Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71:3540. PMID: 21430066.
-
Koyama, S. et al. (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7:10501. PMID: 26883990.
-
Du, W. et al. (2017) TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 18:645. PMID: 28300768.
-
Dardalhon, V. et al. (2010) Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J. Immunol. 185:1383. PMID: 20574007.
