Apoptosis Signaling
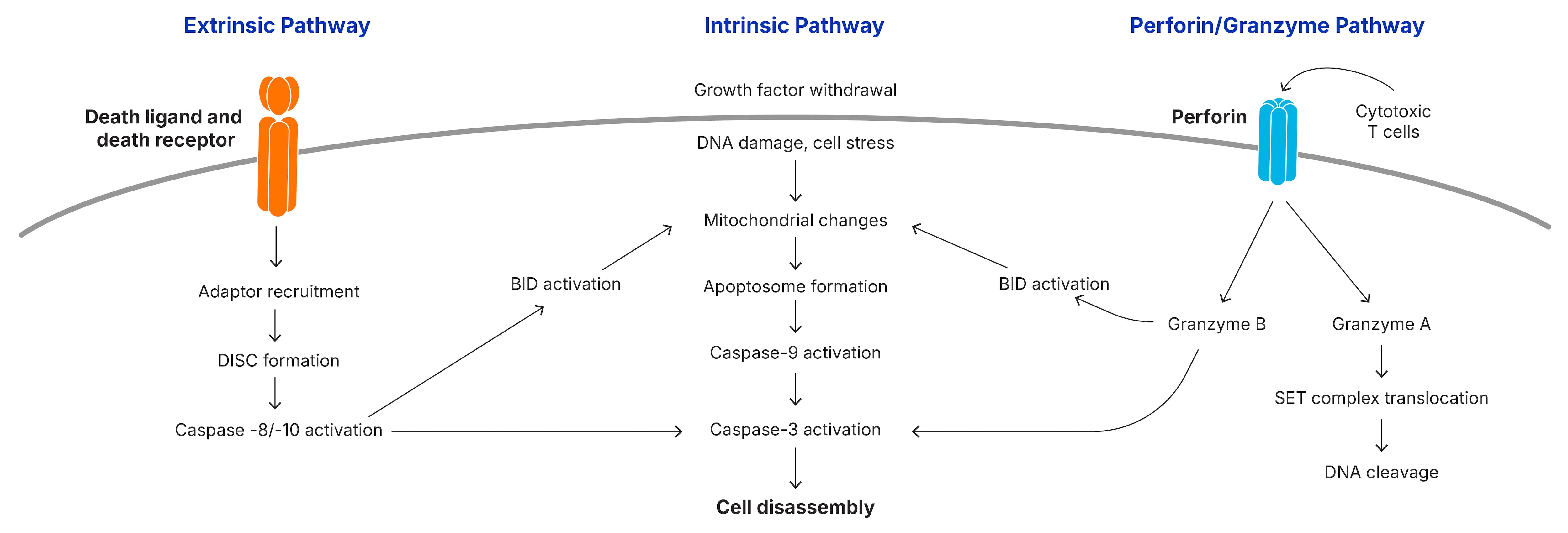
Apoptosis is initiated by the intrinsic or extrinsic signaling pathways, or in some cases by the perforin/granzyme B pathway and results in the activation of the caspase cascade. Multiple protein families are involved in the signaling pathways that promote or inhibit caspase activation.
Initiation of Apotosis Signaling Network
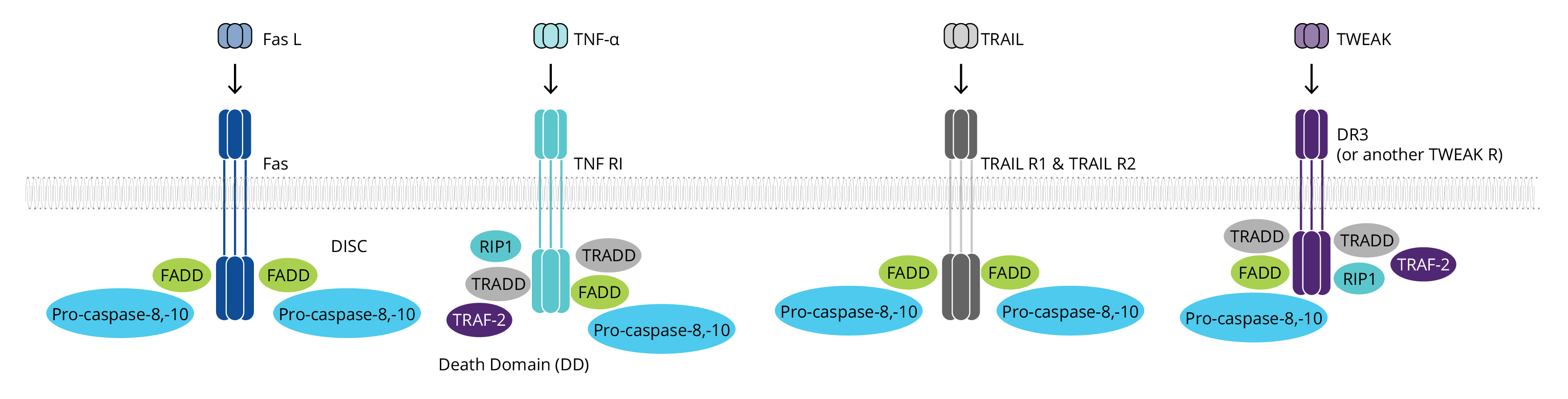
Extrinsic/Death Receptor Pathway
The extrinsic pathway begins with the binding of a ligand to one of several death receptors, all members of the TNF receptor superfamily. This interaction triggers receptor oligomerization and the recruitment of adaptor proteins containing death domains (DD), such as TRADD and FADD. The resulting complexes bind and activate pro-caspases-8 and pro-caspases-10. The ligands including FASL, TNF-α, TRAIL and TWEAK may be anchored in the plasma membrane of neighboring cells or can act as soluble cytokines.
Intrinsic/Mitochondrial Pathway
The intrinsic pathway of caspase activation can be initiated by a variety of unrelated factors, including DNA damage, growth factor withdrawal, loss of contact with the extracellular matrix, or exposure to glucocorticoids. These stimuli induce signaling cascades that result in the loss of mitochondrial integrity, release of cytochrome c, and the subsequent activation of caspase-9.
Mitochondrial integrity is regulated by the family of Bcl-2 proteins, a group of more than 20 structurally related proteins that contain one to four Bcl-2 homology (BH) domains. Bcl-2 proteins are divided into 3 distinct subfamilies based on the presence of BH domains and their ability to either promote or inhibit apoptosis. The balance between pro- and anti-apoptotic family members determines whether the cell survives an apoptotic insult or undergoes cell death.
Classification of Mammalian Bcl-2 Family Members
| Function | BH domain | Proteins* |
|---|---|---|
| Pro-apoptotic | BH multidomain | BAK, Bax, Bcl-rambo, Bcl-xs, BOK |
| Pro-apoptotic | BH3-only** | Bad, BID, Bik/Blk, BIM, Bmf, Hrk/DP5, Noxa, PUMA |
| Anti-apoptotic | BH multidomain | Bcl-2, Bcl-10, BclA1, Bcl-B, Bcl-w, Bcl-xL, Mcl-1 |
What characteristics of Bcl-2 proteins are commonly exploited in immunoassays for studying apoptotic signaling pathways?
- Protein quaternary structure
- Protein conformation
- Expression levels
- Post-translational modifications
- Cellular localization
For example, cleavage of cytoplasmic BID during apoptosis results in the migration and insertion of the C-terminal fragment, tBID (truncated BID), into the mitochondrial membrane. Likewise, the pro-apoptotic protein, Bax, is predominantly found as an inactive monomer in the cytosol in healthy cells, but oligomerizes and translocates to the mitochondria upon activation.
Granzyme B Pathway
During an immune response to viruses and cellular transformation, cytotoxic cells including cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells identify affected cells and release serine proteases known as granzymes into the cytosol of targeted cells. Granzyme B triggers apoptosis either by cleaving BID to induce mitochondrial outer membrane permeabilization (MOMP) or by directly processing effector caspases.
| Granzyme* | A | B | H / C | K | M |
|---|---|---|---|---|---|
| Activity | Tryptase | Chymase | Chymase | Tryptase | Metase |
| Predicted Specificities | Arg/Lys/Phe | Asp/Asn/Met/Ser | Phe/Leu | Arg/Lys/Phe | Met/Leu |
| Chromatin Condensation | Yes | Yes | Yes | Yes | Yes |
| DNA Fragmentation | No | Yes | No | No | No |
| DNA Nicks | Yes | No | Yes | Yes | Not Determined |
| Caspase Dependent | No | Yes | No | No | Yes |
| Inhibition of Bcl-2 | No | Yes | Not Determined | Yes | No |
| Cytochrome C Release | No | Yes | Yes | No | No |
| Mitochondrial Swelling | Yes | Yes | Yes | Yes | Yes |
Granulysin, a component of the dense core of cytotoxic granules, is known for its anti-microbial and anti-cancer activity. It can initiate cytochrome c release mediated apoptotic cell death. Some other granule-associated proteins include additional apoptosis mediators, pro-enzyme activators, and proteins with the ability to inhibit self-destruction of the effector cells (see table below).
Cytolytic Granule Contents and Their Functions
| Protein | Function |
|---|---|
| Perforin | A membrane pore forming protein which facilitates granzyme entry into the cytoplasm of target cells |
| Calreticulin | An ER resident protein which binds Perforin and potentially inhibits Perforin-mediated damage to the effector cell |
| Cathepsin B | A lysosomal cysteine protease which appears on the cell surface following degranulation and may inhibit Perforin-mediated self-destruction |
| Cathepsin C | A lysosomal proteinase which processes granzyme pro-enzymes |
| Granulysin | A saposin family protein with cell membrane perturbing potential and the ability to induce apoptosis via release of Cytochrome c |
| Serglycin | An intracellular proteoglycan that binds granzymes (non-covalently) and facilitates granzyme exocytosis from killer cells |
| Chemokines | A family of small cytokines which mediate hemotaxis and/or cell activation |
| LAMPs | Lysosome-associated membrane proteins which negatively regulate auto-digestion and facilitate phagosome-lysosome fusion |
| WIP | Vesicular protein critical for organization of the actin cytoskeleton and cytolytic granule transport |
| FAS Ligand/TNFSF6 | A member of TNF superfamily which acts as a potent inducer of apoptotic cell death |