Immuno-oncology: Leveraging Antibodies to Target Cancer
Antibody-mediated Immunotherapy | Immune Checkpoint Blockade | Bispecific Antibodies | Single Domain VHH Antibodies
Immuno-Oncology harnesses, redirects, and boosts the power of the immune system to target cancer. Unlike traditional chemotherapy, which attacks all rapidly dividing cells whether they’re healthy or malignant, immunotherapy is more targeted, utilizing the body’s natural defenses to strategically attack cancer cells. Immunotherapy for cancer is a direct result of advances in technology for recombinant antibody generation, cell and gene therapy engineering, and increasing appreciation of the immune system’s role in cancer prevention and control. It has been successfully deployed in the clinic for hematological malignancies, such as Chronic Lymphocytic Leukemia (CLL), Non-Hodgkin Lymphoma (NHL), and Acute Lymphocytic Leukemia (ALL). While solid tumors have proven more challenging to target, immunotherapy is gaining traction for treatment in some cases. Once considered a last resort treatment, immunotherapy is often the first line of treatment for advanced and metastatic melanoma.
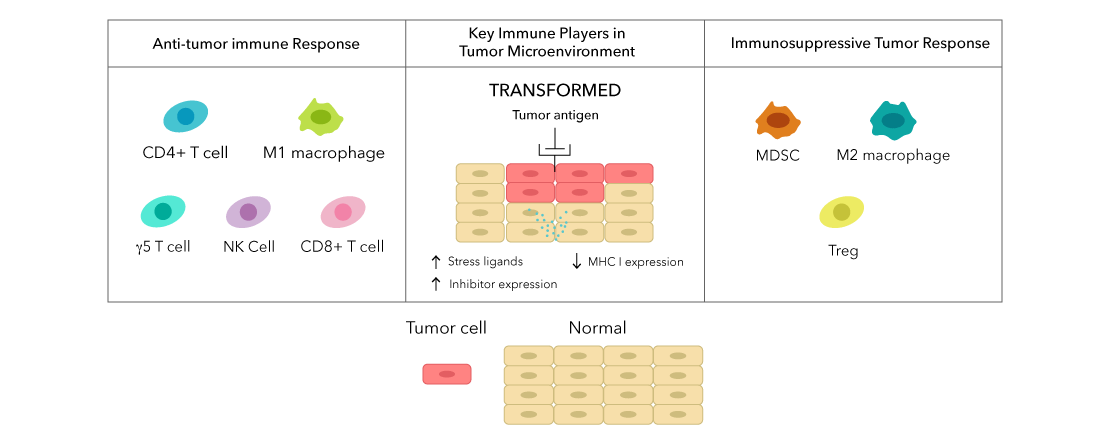
The immune system is involved at every stage of tumor suppression:
- Natural Killer (NK) cells, γδ T cells, and cytotoxic CD8 T cells promote apoptosis of tumor cells.
- Dendritic cells (DCs) prime CD4 and CD8 T cells against tumor-associated antigens (TAAs).
- Macrophages phagocytose dead and dying cells and promote inflammation.
However, during tumorigenesis and disease progression, the tumor actively suppresses this potent immune response by:
- Creating a hypoxic microenvironment, excluding cytotoxic T cells.
- Secreting TGF-beta (TGF-β) and adenosine.
- Promoting formation and recruitment of immunosuppressive cells: T regulatory cells (Tregs), Myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs).
- Upregulating inhibitory ligands to prevent cell-mediated cytotoxicity.
In summary, the immune system has a potent anti-tumor response, and the tumor has a potent anti-immune response. Immunotherapy is what helps tip the balance in favor of the anti-tumor immune response.
Antibody-Mediated Immunotherapy
One of the primary strategies to boost the immune response to tumors leverages the specificity of antibodies. According to the Cancer Research Institute, there are three primary types of antibody-mediated therapies: Monoclonal antibodies (mAbs), Bispecific antibodies, and Antibody-Drug Conjugates (ADCs).
| Monoclonal Antibodies | Bispecific Antibodies | |
|---|---|---|
| Description | Recognizes one antigen | Recognizes two antigens |
| Target (Antigen) |
|
|
| Function |
|
|
Note: More information about ADCs can be found at the Cancer Research Institute.
Immune Checkpoint Blockade
The immune response is tightly regulated by checkpoints, achieving a careful balance between activation and inhibitory receptors. Inhibitory receptors on immune cells are critical to avoid unnecessary damage to the host. Innate cells, like NK cells and γδ T cells, constitutively express inhibitory receptors to prevent killing and phagocytosis of healthy host cells, while α/β T cells upregulate inhibitory receptors soon after activation to regulate expansion and effector function. Tumor cells, like those of non-small cell lung cancer (NSCLC), prostate cancer and melanoma, will upregulate inhibitory ligands to avoid destruction by the immune system.
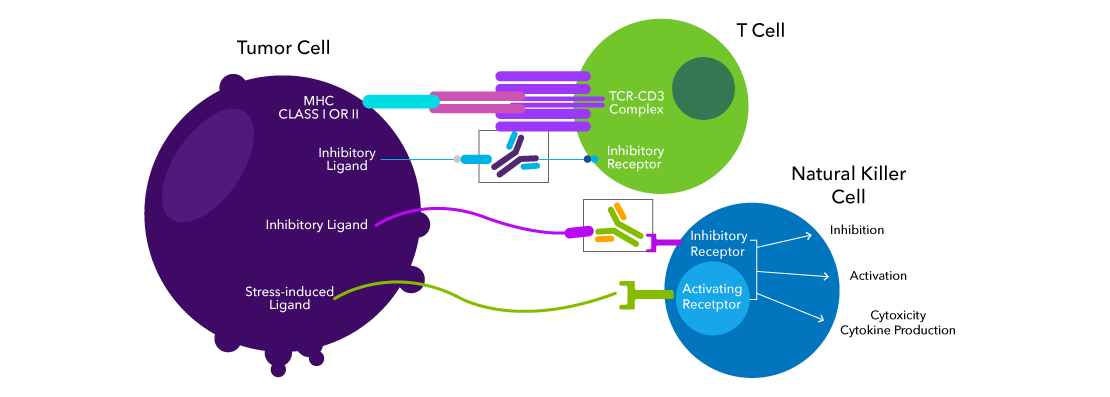
In general, immune checkpoint blockade therapy seeks to disrupt the receptor-ligand interaction between the inhibitory receptor of the immune cells and the ligand expressed on the tumor cells. Checkpoint blockade therapy liberates the immune cells from this inhibition. Ex vivo or in vitro characterization of immune checkpoints is the first step towards development of effective immunotherapy.
In addition to flow cytometry, immunohistochemistry (IHC), and immunocytochemistry/immunofluorescence (ICC/IF), Bio-Techne has a large selection of highly validated immune checkpoint antibodies for blocking and neutralization.
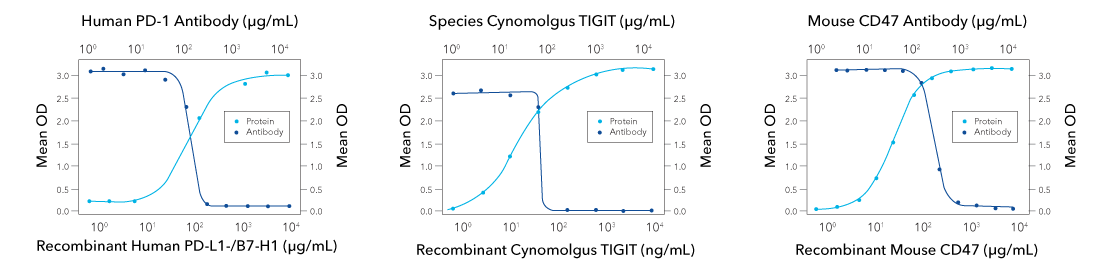
Functional ELISA data showing successful blocking of receptor-ligand interaction with receptor-blocking antibody. Orange line shows recombinant ligand binds to receptor in dose-dependent manner, in the absence of the antibody. (Left) At 0.09-0.72 µg/mL, Mouse Anti-Human PD-1 (1015846) (Catalog # MAB10864) will block 50% of the binding of 5 µg/mL of Recombinant Human PD-L1/B7-H1 Fc Chimera (orange line, Catalog # 156-B7) to immobilized Recombinant Human PD 1 His-tagged Protein (Catalog # 8986-PD) coated at 1 µg/mL (100 µL/well). At 5 µg/mL, this antibody will block >90% of the binding. (Middle) At 70-350 ng/mL, Rabbit Anti-Cynomolgus TIGIT (2629A) (Catalog # MAB10532) will block 50% of the binding of Recombinant Cynomolgus Monkey TIGIT (Catalog # 9380-TG) bound to immobilized Recombinant Human CD155/PVR (Catalog # 2530-CD) coated at 2.5 µg/mL (100 µL/well). (right) At 0.08-0.8 µg/mL, Rat Anti-Mouse CD47 (974222) (Catalog # MAB18661) will block 50% of the binding of 0.25 µg/mL of Recombinant Mouse CD47 Fc Chimera (orange line, Catalog # 1866-CD) to immobilized Recombinant Mouse SIRP alpha /CD172a Fc Chimera (Catalog # 7154-SA) coated at 1 µg/mL (100 µL/well). At 5 µg/mL, this antibody will block >90% of the binding.
Current and Emerging Targets for Immune Checkpoint Blockade
The first two immune checkpoint blockade therapies approved for use in humans target the CTLA-4/CD80-CD86 and PD-1/PD-L1 axes, both members of the B7-CD28 families of immune checkpoints. The B7 family proteins are expressed on surface of antigen presenting cells (APCs) and tumor cells and interact with receptors of the CD28 family of proteins on immune cells. Members of these families are required for both co-stimulatory and co-inhibitory functions of T cells. Many other members of B7-CD28 protein families are under investigation as additional targets for immunotherapy.
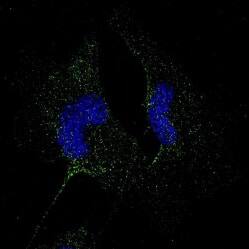
PD-L1 is clinical target for immune checkpoint blockade. Highly validated anti-PD-L1 antibody cross-reactive in human, mouse, and rat tissues. ICC/IF of U-251 cell line from malignant glioblastoma. Cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized in 0.05% Triton X-100 in PBS for 5 minutes. The cells were incubated with Rabbit Anti-Human/Mouse/Rat PD-L1 (Catalog # NBP1-76769) at 1 μg/mL overnight at 4°C and detected with an anti-rabbit DyLight 488 (green) at a 1:1000 dilution for 60 minutes. Nuclei were counterstained with DAPI (blue). Cells were imaged using a 100X objective and digitally deconvolved.
B7 Family Immune Checkpoints
| Receptor: On Immune Cell | Ligand: On Tumor Cell |
|---|---|
| PD-1 | PD-L1, PD-L2 |
| CTLA-4 | CD80, CD86 |
| Unknown Receptor | B7-H3/CD276 |
| Unknown Receptor | B7-H4 |
| PSGL-1, Unknown alternate receptor | VISTA/B7-H5 |
| VISTA/B7-H5 | VSIG-3 |
| KIR3DL3 | B7-H7/HHLA2 |
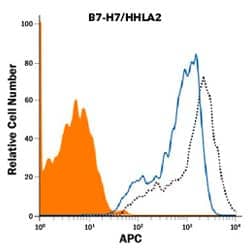
Blocking/Neutralization of HHLA-2 in a functional flow cytometry assay. Briefly, HEK293 human embryonic kidney cell line was transfected with human B7-H7/HHLA-2 and stained with Biotinylated Recombinant Human TMIGD2/CD28H (10 ng/mL, Catalog # 8316-TR), followed by Streptavidin-APC (Catalog # F0050-NOV) (black, dotted line). Binding is completely blocked (orange histogram) by 2.5 μg/mL of Mouse Anti-Human B7-H7/HHLA2 (907812) (Catalog # MAB80841). Mouse IgG1 Isotype Control (Catalog # MAB002) at 2.5 μg/mL was used as a control (blue line).
A 2018 study found that only about 43% of cancer patients are eligible for checkpoint blockade therapy, and the frequency of patients who respond is around 12%. Because such a small percentage of patients respond to current checkpoint blockade, additional checkpoints, beyond the B7 family, are of intense interest, including other inhibitors of cytotoxicity, like TIM-3 and TIGIT. Tumor cells can also upregulate ligands to inhibit phagocytosis by patrolling macrophages and myeloid cells. These ligands, such as CD24 and CD47, are expressed on all mammalian cells and are a “don’t eat me” signal to the innate immune system.
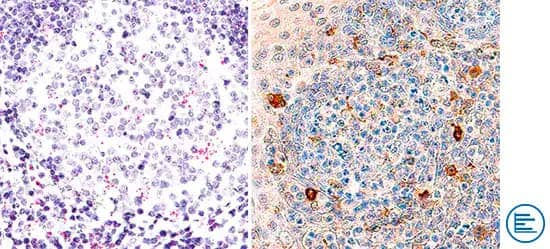
Orthogonal Strategies Validation. TIM-3 is an inhibitory receptor expressed on the surface of T cells and NK cells. TIM-3 mRNA was detected in formalin-fixed paraffin-embedded tissue sections of human tonsil probed with ACD RNAScope® Probe HAVCR2 (ACD Catalog # 560681) and stained using ACD RNAScope® 2.5 HD Detection Reagents-Red (right image, ACD Catalog # 32260). Adjacent tissue section was processed for immunohistochemistry using Goat Anti-Human TIM-3 Antibody (R&D Systems Catalog # AF2365) followed by incubation with the Anti-Goat IgG VisUCyte HRP Polymer Antibody (R&D Systems, Catalog # VC004) and DAB chromogen (right image, yellow-brown). Tissues were counterstained with hematoxylin (blue; Catalog # 5222).
| Receptor: On Immune Cell | Ligand: On Tumor Cell | |
|---|---|---|
| Inhibition of Cytotoxicity | LAG-3 | MHC-II, Galectin-3, LSECtin/CLEC4G, FGL-1 |
| TIGIT | CD155 (PVR) , CD112 (PVRL2) | |
| TIM-3 | Galectin-9, HMGB-1 | |
| ILT2 | HLA-G | |
| NKG2A | HLA-E | |
| Inhibition of Phagocytosis | Siglec-10 | CD24 |
| SIRP-alpha | CD47 |
Learn about more immune checkpoint targets with Bio-Techne’s eBook, Current and Emerging Immune Checkpoint Targets for Immuno-Oncology Research.
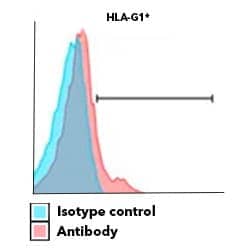
HLA-G expression contributes to immunomodulatory function of human keratinocytes. Surface expression of HLA-G determined by flow cytometry of human keratinocytes stained with Alexa Fluor 700 Conjugated Mouse Anti-Human HLA-G (87G) (Catalog # NBP1-43123AF700) (red histogram) or isotype control (blue histogram). Image adapted from Mestrallet G, Auvré F, Schenowitz C, Carosella ED, LeMaoult J, Martin MT, Rouas-Freiss N, Fortunel NO. Human Keratinocytes Inhibit CD4+ T-Cell Proliferation through TGFB1 Secretion and Surface Expression of HLA-G1 and PD-L1 Immune Checkpoints. Cells. 2021 Jun 8;10(6):1438. doi: 10.3390/cells10061438. PMID: 34201301; PMCID: PMC8227977. License provided by CC-BY.
Efficacy of immune checkpoint blockade is determined by several factors: expression of inhibitory receptors by tumors, penetration of therapeutic antibodies into solid tumors, and accessibility of the tumor to immune cells. Due to redundancy of inhibitor expression (e.g. both LAG-3 and PD-1 expressed on the same cell) and the complexity of the tumor microenvironment (TME), combination immune checkpoint blockade might be required to achieve optimal anti-tumor immunity. Combination checkpoint blockade could include two or more co-inhibitory receptors or a multipronged approach targeting an inhibitory receptor and phagocytosis inhibitor, like CD47. Combination blockade can be achieved with a cocktail of monoclonal antibodies or an engineered bispecific antibody.
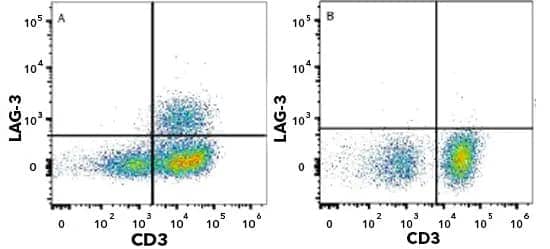
LAG-3 inhibitory receptor expressed on the surface of stimulated T cells. Human peripheral blood mononuclear cells (PBMCs) were either untreated (bottom panel) or treated with 5 μg/mL PHA (top panel). PBMCs were stained with Mouse Anti-Human LAG-3 (1009611 ) (Catalog # MAB23195) followed by Allophycocyanin (APC)-conjugated Anti-Mouse IgG Secondary Antibody (Catalog # F0101B) and PE-conjugated Mouse Anti-Human CD3 epsilon (UCHT1) (Catalog # FAB100P). Quadrant markers were set based on isotype control antibody staining (Catalog # MAB003). CD3 clone UCHT1 was used by HCDM in HLDA Workshop to establish CD designation.
Bispecific Antibodies
Advances in antibody engineering have led to the development of bispecific antibodies. As indicated by their name, the variable regions of these antibodies recognize two different antigens.

Ig-Like
| Structure | Complete heavy chain (Hc) and light chain (Lc) from two monoclonal antibodies |
| Function | Binds two antigens and constant region (Fc) can engage Fc receptors (FcRs) on DCs, macrophages, and NK cells |
| Advantages |
Can engage FcRs for additional functionality:
|

F(ab)2
| Structure | Full length antigen binding fragments (F(ab)) of two monoclonal antibodies, chemically linked |
| Function | Bi-functional: binds two antigens with no functional Fc domain |
| Advantages |
|
ScFv
| Structure | Single chain variable fragments (scFv) from heavy and light chain of two monoclonal antibodies |
| Function | Bi-functional: binds two antigens with no functional Fc domain |
| Advantages |
|
Blue and green represent two different antigen specificities. Only one example of F(ab)2 and ScFv fragments structures are shown.
For cancer therapy, bispecific antibodies are utilized in two ways:
- Simultaneous blockade of two immune checkpoints.
- Redirection of cytotoxic cells to interact with tumor cell.
Spotlight: Redirection of Cytotoxic Cells
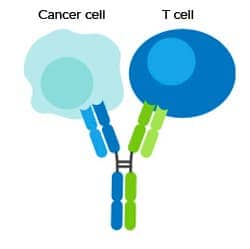
Bispecific antibodies to redirect cytotoxic cells are of the greatest interest for cancer immunotherapy. The primary goal of this type of immunotherapy is to bring the cytotoxic cell, either T cell or NK cell, into proximity with the tumor cell to facilitate killing. Ideally, the bispecific antibody is engineered to activate the cytotoxic cell via CD3 for T cells and CD16 for NK cells and bind to a uniquely expressed TAA.
| Tumor Associated Antigen (TAA) | Cancer Type |
|---|---|
| Carcinoembryonic Antigen (CEA) | Colorectal Cancer |
| CD33/Siglec-3 | Acute Myeloid Leukemia (AML), Lymphomas |
| EGFR | Breast Cancer, Pancreatic Cancer |
| HER2/ERBB2 | Breast Cancer, Prostate Cancer |
| EpCAM | Breast Cancer, Colorectal Cancer, Endometrial Cancer, Ovarian Cancer, Pancreatic Cancer, Prostate Cancer |
| MUC1 | Breast Cancer, Ovarian Cancer, Prostate Cancer |
| Prolactin Receptor (PRLR) | Breast Cancer |
| VEGFR1 | Breast cancer |
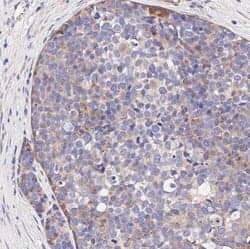
VEGFR1/Flt-1 expression is confined to cancer cells and is not expressed on tumor stromal cells. IHC-Paraffin (IHC-P) analysis of a formalin-fixed paraffin-embedded (FFPE) human breast carcinoma tissue section using 1:500 dilution of Rabbit Anti-VEGFR1/Flt-1 Polyclonal Antibody (Catalog # NB100-527). The assay involved 20 minutes of heat induced antigen retrieval (HIER) with 10mM sodium citrate buffer (pH 6.0) and endogenous peroxidase quenching using peroxide block. A polymer refined detections system and DAB were used for signal detection which followed counterstaining with hematoxylin. Staining was performed with Histowiz.
Often, TAAs targeted by antibody-therapy are not unique to the tumor cells and are also expressed on healthy cells. However, TAAs are frequently overexpressed on tumor cells compared to healthy cells, providing an opportunity to exploit advanced antibody engineering technology. To limit the redirection of cytotoxic cells to healthy, non-malignant cells, bispecific antibodies provide a distinct advantage over traditional antibody therapies due to an engineering technique often referred to as affinity tuning. Affinity tuning involves selecting antibodies with certain binding properties, or affinity, for a particular antigen. During an immune response in vivo, antibodies often undergo a process called affinity maturation where high affinity antibodies are selected in the germinal center reaction. Researchers can simulate this process in vitro by introducing targeted mutations of the variable region and measuring antibody affinity via surface plasmon resonance (SPR).
An affinity-tuning approach allows for selection of bispecific antibodies with weak antibody binding (low affinity) to the activating receptor (CD3 or CD16) and strong binding (high affinity) to the TAA, such as EpCAM. This increases the likelihood that the antibody will be bound to a tumor cell before it activates the cytotoxic cell, enhancing efficacy and reducing non-specific activation.
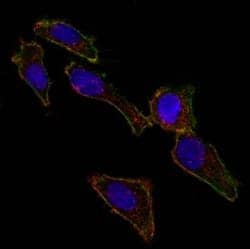
Bio-Techne antibodies are validated for a variety of applications, including ICC/IF. Confocal immunofluorescent analysis of ovarian cancer cell line SK-OV-3 using Alexa Fluor 488-conjugated Mouse Anti-Human EpCAM (EGP40/826) (Catalog # NBP2-44643) (green). F-actin filaments were labeled with DyLight 554 Phalloidin (red). DAPI was used to stain the cell nuclei (blue).
As an additional challenge, antibodies directed against CD3 will indiscriminately target both CD8+ cytotoxic T cells and CD4+ T regulatory cells, which could promote an immunosuppressive TME. Though currently approved therapies target CD3 and TAA, researchers are also exploring bispecific antibodies targeting NK cell markers, like CD16A.
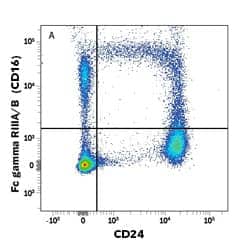
CD16 is expressed on the surface of human NK cells. Human peripheral blood mononuclear cells (PBMCs) were stained with PE-conjugated Mouse Anti-Human Fc gamma RIII/CD16 (245536) (Catalog # FAB2546P) and APC-conjugated Mouse Anti-Human CD14 (134620) (Catalog # FAB3832A). Quadrant markers were set based on isotype control antibody staining (Catalog # IC003P).
Limitations and Future Directions of Antibody-Based Immunotherapy
With the implementation of antibody-based therapies, there have been significant advancements in treatment of high mortality malignancies such as metastatic NSCLC and melanoma. However, there are still significant limitations of antibody-mediated immunotherapy including:
- Limited penetration into solid tumors.
- Ig-like antibodies with Fc domains can be immunogenic.
- Checkpoint blockade is only effective for “hot” tumors with immune activity and expression of inhibitory ligands.
Nanobodies® with single domain VHH antibodies, like Bio-Techne’s LlaMABodiesTM, can target hard to reach epitopes and may have a role in future immunotherapies.
Moore GL, Lee S-H, Schubbert S, Miranda Y, Rashid R, Pong E, et al. Tuning T Cell Affinity Improves Efficacy and Safety of Anti-CD38 × Anti-CD3 Bispecific Antibodies in Monkeys - a Potential Therapy for Multiple Myeloma. Blood 2015;126:1798–1798. https://doi.org/10.1182/BLOOD.V126.23.1798.1798.
Wang W, Guo H, Geng J, Zheng X, Wei H, Sun R, et al. Tumor-released Galectin-3, a Soluble Inhibitory Ligand of Human NKp30, Plays an Important Role in Tumor Escape from NK Cell Attack. J Biol Chem 2014;289:33311. https://doi.org/10.1074/JBC.M114.603464.
Lum LG, Thakur A, Choi M, Deol A, Kondadasula V, Schalk D, et al. Clinical and immune responses to anti-CD3 x anti-EGFR bispecific antibody armed activated T cells (EGFR BATs) in pancreatic cancer patients. Https://DoiOrg/101080/2162402X20201773201 2020;9:. https://doi.org/10.1080/2162402X.2020.1773201.
Müller P, Rios-Doria J, Harper J, Cao A. Combining ADCs with Immuno-Oncology Agents. Cancer Drug Discov Dev 2018:11–44. https://doi.org/10.1007/978-3-319-78154-9_2.
Chauvin J-M, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer 2020;8:e000957. https://doi.org/10.1136/JITC-2020-000957.
Barkal A, Brewer R, Markovic M, Kowarsky M, Barkal S, Zaro B, et al. CD24 signaling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019;572:392–6. https://doi.org/10.1038/S41586-019-1456-0.
Strome AL, Zhang X, Strome SE. The evolving role of immuno-oncology for the treatment of head and neck cancer. Laryngoscope Investig Otolaryngol 2019;4:62–9. https://doi.org/10.1002/LIO2.235.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 2019 201 2019;20:7–24. https://doi.org/10.1038/s41577-019-0210-z.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 2021 218 2021;21:485–98. https://doi.org/10.1038/s41577-020-00490-y.
DeNardo DG, Ruffell B. Macrophages as regulators of tumor immunity and immunotherapy. Nat Rev Immunol 2019 196 2019;19:369–82. https://doi.org/10.1038/s41577-019-0127-6.
Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov 2019 188 2019;18:585–608. https://doi.org/10.1038/s41573-019-0028-1.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020 2011 2020;20:651–68. https://doi.org/10.1038/s41577-020-0306-5.
Marshall HT, Djamgoz MBA. Immuno-Oncology: Emerging Targets and Combination Therapies. Front Oncol 2018;0:315. https://doi.org/10.3389/FONC.2018.00315.
Nanobodies is a registered trademark of Ablynx N.V.